Possible applications
These products are recommended by experts for the treatment of herbes, as they have the ability
- prevent the formation of new sites of infection;
- reduce the possibility of spreading infection through internal organs;
- speed up the healing of ulcers;
- reduce pain in the acute phases of the disease;
- stimulate the body's immune system.
The variety of forms of the drug containing acyclovir, which are offered by the pharmaceutical industry, makes it possible to use the drug both for local treatment and in a system with other formulations.
Overdose
Acyclovir is only partially absorbed from the gastrointestinal tract. As a rule, no toxic effects have been reported with a random single dose of acyclovir up to 20 g. With repeated oral doses over several days of doses exceeding the recommended ones, disturbances from the gastrointestinal tract (nausea, vomiting) and nervous system (headache and confusion) were observed. Sometimes neurological effects such as seizures and coma may occur.
Patients require careful medical monitoring to identify possible symptoms of intoxication. Acyclovir is eliminated from the body by hemodialysis, so hemodialysis can be used to treat overdose.
Dosage forms
Products containing the active substance are produced by the following companies:
- in tablets;
- in powders intended for the preparation of solutions used for intravenous drip administration of the drug;
- in a thick cream or ointment consistency.
The use of the product, regardless of the release form, does not impose any age restrictions and is generally recommended based on the indications in each individual case. However, the use of compositions used to prepare solutions for drip administration implies hospital treatment, as it requires compliance with certain precautions.
Drugs produced in the form of creams, ointments and tablets are recommended for outpatient treatment.
Side effect
The frequency categories of adverse reactions listed below are estimates. For most adverse reactions, the necessary data to determine the frequency of occurrence are not available. In addition, the incidence of adverse reactions may vary depending on the indication.
The adverse reactions presented below are listed according to their frequency of occurrence, defined as follows: very common (>1/10), common (>1/100 and <1/10), uncommon (>1/1000 and <1/100 ), rare (>1/10,000 and <1/1000), very rare (<1/10,000).
Frequency of occurrence of adverse reactions
Blood and lymphatic system disorders
Very rare: anemia, leukopenia, thrombocytopenia.
Immune system disorders
Rarely: anaphylaxis.
Nervous system and mental status disorders
Common: headache, dizziness.
Very rare: agitation, confusion, tremor, ataxia, dysarthria, hallucinations, psychotic symptoms, convulsions, drowsiness, encephalopathy, coma.
Typically, these side effects were observed in patients with renal failure or in the presence of other precipitating factors and were mainly reversible (see section "Special instructions"
).
Respiratory, thoracic and mediastinal disorders
Rarely: shortness of breath.
Gastrointestinal disorders
Common: nausea, vomiting, diarrhea, abdominal pain.
Disorders of the liver and biliary tract
Rare: reversible increase in the concentration of bilirubin and liver enzymes in the blood.
Very rare: hepatitis, jaundice.
Skin and subcutaneous tissue disorders
Common: itching, rash, including photosensitivity.
Uncommon: urticaria, rapid diffuse hair loss.
Since rapid diffuse hair loss is observed in various diseases and during therapy with many drugs, its connection with the use of acyclovir has not been established.
Rarely: angioedema.
Very rare: toxic epidermal necrolysis, exudative erythema multiforme.
Renal and urinary tract disorders
Rarely: increased concentrations of urea and creatinine in the blood serum.
Very rare: acute renal failure, renal colic.
Renal colic may be associated with renal failure and crystalluria.
General and administration site disorders
Common: fatigue, fever.
Recommendations for use
Indications for the use of formulations containing acyclovir are:
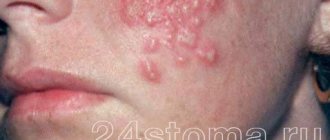
- eye diseases caused by herbes simplex virus;
- herbes of the red border of the lips and skin tissues;
- viral stomatitis of the oral cavity;
- genital herpes;
- hepatic encephalitis.
For successful treatment of the disease, experts recommend:
- 5 percent creams, effective for external use;
- 5 percent ointments;
- ophthalmic 3 percent formulations;
- tablet forms, dosed at 200 and 400 mg;
- powders, for solutions, doses from 200 mg to 1 g.
Acyclovir - instructions for use
When treating stomatitis caused by the herbes virus, it is customary to use acyclovir tablets, since creams and ointments are not able to have the desired effect due to the impossibility of fixing fat-based medications in the oral cavity. Therefore, such compositions are recommended for use when infection spreads in the skin tissues.
The course of the disease in severe form often requires the administration of drug solutions intravenously.
Acyclovir tablets
The course of taking tablets is from five to ten days, during which the drug is taken 4-5 times a day. For both adult patients and children starting from two years of age, the dosage of the drug is 200 mg at a time, and for children under two years of age the dose is halved, which is 100 mg.
Diseases that occur in severe forms often require an increase in dosage and therefore the drug can be prescribed in an amount of 400 mg per dose.
It should be taken into account that tablets should be taken during the daytime, which allows the drug not to settle in the kidneys and not cause side effects in the genitourinary system of the body. In addition, it is recommended to increase the volume of fluid consumed by the patient, which helps reduce the concentration of acyclovir in the urine and prevent the development of kidney disorders.
Treatment of the so-called “chickenpox” with drugs containing acyclovir is not recommended for children, since their age allows them to tolerate this disease in a milder form than adults.
Powder forms of acyclovir
Compositions for administering the drug through a dropper are prepared on the basis of physiological solution, for which bottles containing a certain amount of the drug are filled with liquid, according to the instructions, shaken thoroughly until the suspended particles are completely dissolved, and the finished solution is poured into containers for drip infusions.
Before the first use of the composition, it is necessary to test the tolerability of the drug, which allows you to avoid allergic reactions of the body when using it. The test involves applying the drug to a shallow scratch made with a needle on the inside of the forearm and observing for several minutes. If an area of inflammation forms around the damaged surface, the use of acyclovir should be avoided.
Drip administration of the drug is justified by the fact that the content of the drug in the patient’s blood should increase gradually, since otherwise this procedure can provoke complications. Therefore, one-time procedures are carried out within an hour, and sometimes longer.
Acyclovir ointment - instructions for use
Acyclovir ointment, the use of which is recommended for the local treatment of ailments caused by the herbes virus, is offered in tubes of 2, 5 or 10 g, the content of the active substance in these compositions is exactly 5%, which makes it possible to use the drug in the initial phase of the disease. Thus, products in the form of ointments and creams are recommended to be used at the first signs of the disease:
· when itching occurs;
· minor pain.
The procedures should be continued for 5-10 days, applying the composition to the skin, without rubbing the product, into the damaged area.
Acyclovir helps reduce the intensity of skin lesions, promoting rapid healing and crust formation. However, the crusts that form when using the drug should not be removed independently, since such an initiative significantly reduces the effectiveness of treatment and provokes infection of others with the herbes virus.
In addition, the use of the drug for medicinal purposes does not prevent the spread of the disease through sexual contact.
Eye ointment containing acyclovir
Such products are commercially produced in small tubes containing up to five grams of the drug, with an acyclovir concentration of 3%.
The use of the composition involves placing the product behind the lower eyelid, a thin strip up to one centimeter long, which helps the composition spread across the cornea and absorption into the surrounding tissues.
It is recommended to use the drug up to 5 times a day, for 5-10 days, however, if signs of intolerance occur, you must see a specialist and change the medicine.
Cost of drugs
Drugs in various forms are produced by both domestic and foreign pharmaceutical companies, therefore the choice of products that include acyclovir is quite diverse. In addition, the cost of these antiviral agents is affordable.
Thus, acyclovir in tablets, the price of which from domestic manufacturers does not exceed 250 rubles, allows people of any income to purchase and use an effective remedy.
Drugs produced in the form of ointments and creams are also not very expensive, which makes it possible to successfully cope with the disease in its initial stages without resorting to hospital treatment.
Powder forms of acyclovir, used for drip administration by professional medical workers, are produced mainly abroad, which affects the cost, but their use helps to cure severe viral infections.
Description:
Round flat-cylindrical tablets of white or almost white color with a chamfer and a score.
Pharmacological group:
antiviral agent.
ATX code:
J05AB01
pharmachologic effect
Pharmacological properties
Pharmacodynamics
Mechanism of action
Acyclovir
is a synthetic analogue of a purine nucleoside that has the ability to inhibit in vitro and in vivo human herpes viruses, including herpes simplex virus (HSV) types 1 and 2, varicella zoster virus and herpes zoster virus (Varicella zoster virus (Varicella zoster virus). VZV), Epstein-Barr virus (EBV) and cytomegalovirus (CMV). In cell culture, acyclovir has the most pronounced antiviral activity against HSV-1, followed in descending order of activity by HSV-2, VZV, EBV and CMV.
The inhibitory effect of acyclovir on herpes viruses (HSV-1, HSV-2, VZV, EBV and CMV) is highly selective. Acyclovir is not a substrate for the thymidine kinase enzyme in uninfected cells, therefore acyclovir is of low toxicity to mammalian cells. However, thymidine kinase of cells infected with HSV, VZV, EBV and CMV viruses converts acyclovir into acyclovir monophosphate, a nucleoside analogue, which is then sequentially converted into diphosphate and triphosphate under the action of cellular enzymes. The incorporation of acyclovir triphosphate into the viral DNA chain and subsequent chain termination blocks further replication of the viral DNA.
In patients with severe immunodeficiency, long-term or repeated courses of acyclovir therapy may lead to the emergence of resistant strains, so further treatment with acyclovir may be ineffective. Most isolated strains with reduced sensitivity to acyclovir had a relatively low content of thymidine kinase, as well as a disorder in the structure of the viral thymidine kinase or DNA polymerase. In vitro exposure of herpes simplex virus (HSV) strains to acyclovir may also result in the formation of strains that are less sensitive to it. A correlation has not been established between the sensitivity of herpes simplex virus (HSV) strains to acyclovir in vitro and the clinical effectiveness of the drug.
Pharmacokinetics
Suction
Acyclovir is only partially absorbed from the intestine. After taking 200 mg of acyclovir every 4 hours, the mean maximum steady-state plasma concentration (Cssmax) was 3.1 μM (0.7 μg/ml), and the mean steady-state minimum plasma concentration (Cssmin) was 1.8 μM (0 .4 µg/ml). When administered orally 400 mg and 800 mg of acyclovir every 4 hours, Cssmax was 5.3 µM (1.2 µg/ml) and 8 µM (1.8 µg/ml), respectively, and Cssmin was 2.7 µM (0.6 µg /ml) and 4 µM (0.9 µg/ml), respectively.
Distribution
The concentration of acyclovir in the cerebrospinal fluid is approximately 50% of its concentration in blood plasma.
Acyclovir binds to blood plasma proteins to an insignificant extent (9-33%), so drug interactions due to displacement from binding sites with blood plasma proteins are unlikely.
Removal
In adults, after taking acyclovir orally, the half-life from blood plasma is about 3 hours. Most of the drug is excreted unchanged by the kidneys. The renal clearance of acyclovir significantly exceeds the clearance of creatinine, which indicates that acyclovir is eliminated through not only glomerular filtration, but also tubular secretion. 9-carboxymethoxy-methylguanine is the main metabolite of acyclovir and accounts for about 10-15% of the dose excreted in the urine. When acyclovir was administered 1 hour after taking 1 g of probenecid, the half-life of acyclovir and AUC (area under the concentration-time pharmacokinetic curve) increased by 18 and 40%, respectively.