Sodium fluoride
| Names | |
| Pronunciation | / ˌ s oʊ d I ə m e l ʊər aɪ d / [1] |
| IUPAC name Sodium fluoride | |
| Other names Flocid | |
| Identifiers | |
| Number of CAS |
|
| CHEBY |
|
| CHAMBL |
|
| ChemSpider |
|
| ECHA InfoCard | 100.028.789 |
| EU number |
|
| KEGG |
|
| PubChem C.I.D. |
|
| RTECS number |
|
| UNII |
|
| UN number | 1690 |
| CompTox Dashboard (EPA) |
|
InCHI
| |
| Characteristics | |
| Chemical formula | NaF |
| Molar mass | 41.988173 g/mol |
| Appearance | White to greenish solid |
| Smell | without smell |
| Density | 2.558 g/cm3 |
| Melting temperature | 993 °C (1819 °F, 1266 K) |
| Boiling point | 1704 °C (3099 °F, 1977 K) |
| Solubility in water | 36.4 g/l (0°C); 40.4 g/l (20 °C); 50.5 g/l (100 °C) [2] |
| Solubility | slightly soluble in ammonia, slightly soluble in alcohol, acetone, SO 2, dimethylformamide |
| Gas pressure | 1 mmHg Art. At 1077 C ° [3] |
| Magnetic susceptibility (χ) | −16.4 10 −6 cm 3 / mol |
| Refractive index ( n D) | 1,3252 |
| Structure | |
| Crystal structure | Cubic |
| Lattice constant | A = 462 pm |
| Molecular form | Octahedral |
| Thermochemistry | |
| Heat capacity ( C ) | 46.82 J/mol K |
| Standard molar entropy ( S o 298) | 51.3 J/mol K |
| Std formation enthalpy (Δ F H ⦵ 298 ) | -573.6 kJ/mol |
| Gibbs free energy (Δ f G ˚) | -543.3 kJ/mol |
| Pharmacology | |
| ATC code | A01AA01 (WHO) A12CD01 (WHO), V09IX06 (WHO) (18 F) |
| Dangers | |
| MSDS | [4] |
| GHS Pictograms | |
| GHS signal word | Danger |
| GHS Hazard Statements | H301, H315, H319, H335 [4] |
| NFPA 704 (fire diamond) | 0 3 0 |
| Flash point | Incombustible |
| Lethal dose or concentration (LD, LC): | |
| LD 50 (average dose) | 52–200 mg/kg (orally rats, mice, rabbits) [6] |
| NIOSH (US Health Exposure Limits): | |
| PEL (Permissible) | TWA 2.5 mg/m3 [5] |
| REL (recommended) | TWA 2.5 mg/m3 [5] |
| IDLH (Imminent Hazard) | 250 mg/m3 (as F) [5] |
| Related compounds | |
| Other anions | Sodium chloride Sodium bromide Sodium iodide Sodium astatide |
| Other cations | Lithium fluoride Potassium fluoride Rubidium fluoride Cesium fluoride France fluoride |
| Related compounds | TASF reagent |
| Unless otherwise stated, data is for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| N check (what is there?)YN | |
| Links to infoboxes | |
Sodium fluoride
(
NaF
) is an inorganic compound with the formula NaF. It is used in trace amounts in the fluoridation of drinking water, toothpaste, in metallurgy as a flux, and is also used in pesticides and rat poison. It is a colorless or white solid, readily soluble in water. It is a common source of fluoride in the production of pharmaceuticals and is used to prevent tooth decay.
In 2022, it was the 247th most commonly prescribed drug in the United States, with more than one million prescriptions. [7] [8]
Receipt in industry
Sodium fluoride is a fairly useful compound, so it is synthesized on an industrial scale. World production is more than 10,000 tons per year. In most cases, the raw materials are hexafluorosilicates, which are also produced artificially. In production, they are subjected to alkaline hydrolysis, as a result of which sodium fluoride is released in the reaction mixture. But it still needs to be separated from impurities of silicon oxide and sodium silicate. This is often done by ordinary filtration.
But hexafluorosilicates, even upon thermal decomposition or upon interaction with sodium carbonate, can produce sodium fluoride. It can also be used in industrial synthesis.
There is also an industrial method for producing sodium fluoride from soda ash (sodium carbonate) and hydrofluoric acid. As a result of their interaction using filtration, it is possible to obtain a technically pure product:
Na2CO3 + HF → 2NaF + CO2 + H2O
Obtained in the laboratory
There are other ways to obtain this compound in the laboratory. The simplest is the reaction of neutralization of sodium hydroxide with hydrofluoric acid. Another option: the interaction of sodium hydroxide with ammonium fluoride. Sodium hydroxide can also produce fluoride when reacted with simple fluorine.
In theory, sodium fluoride can also be obtained from simple substances: sodium and fluorine. This reaction proceeds very violently, although in practice it is carried out very rarely.
F2 + 2Na → 2NaF
Another method of preparation is the thermal decomposition of difluorohydrate and some complex salts. In this case, a product of very high purity is obtained.
Na(HF2) → NaF + HF
When simple fluorine is oxidized with sodium bromate or other sodium-containing oxidizing agents, sodium fluoride can be obtained as a product.
F2 + NaBrO3+ 2NaOH → NaBrO4 + 2NaF + H2O
By reacting boron trifluoride with sodium hydride, this salt can also be obtained.
BF3 + NaOH → Na3BO3 + NaF + H2O
Hazard Class
Sodium fluoride is toxic and poisonous. Sodium fluoride has been assigned hazard class 2. It is especially toxic if it enters the respiratory tract and digestive organs. Poisoning with the substance leads to disruption of the cardiovascular system, surges in blood pressure, and irritation of the gastric tract, including the appearance of ulcers.
Symptoms of sodium fluoride poisoning include:
- nausea, diarrhea and vomiting;
- salivation and lacrimation;
- drop in blood pressure;
- increased body temperature;
- visual impairment.
Sodium fluoride also causes harm when it comes into contact with the skin, which can cause severe chemical burns.
Chemical properties
In aqueous solutions, sodium fluoride dissociates and forms a complex compound.
NaF + 4H2O → [Na(H2O)4]+ + F-
When reacting with hydrofluoric acid, difluorohydrate is formed. But with an excess of hydrogen fluoride, other complex compounds can be formed, which are called sodium hydrofluorides. Their composition may vary depending on the ratio of reagents.
As can be seen from the chemical formula, sodium fluoride is a typical salt, so it undergoes exchange reactions with other salts if the reaction produces a precipitate or gas. When interacting with acids, hydrogen fluoride gas is released. And with lithium hydroxide a precipitate of lithium fluoride is formed.
Sodium fluoride can form other complex salts, depending on the reagents and reaction conditions.
What is fluoride and how does it work?
Fluoride is a form of elemental fluorine, which is normally a toxic gas. Fluoride's chemical reactions with other compounds, such as tin, give it unique properties that increase the element's effectiveness in treating the oral cavity.
Once in the mouth, fluoride is diluted by saliva, depositing as a bacterial plaque on the surface of the teeth. In this state, it directly inhibits the growth of bacteria, so less acid is produced in the mouth. The fluoride stored in the plaque is then released when the bacteria produce enough acid to reduce the acid-base balance in the mouth. When this happens, fluoride diffuses into the tooth through tiny pores in the enamel. Fluoride ions replace the hydroxyl ions in the hydroxyapatite crystals that make up tooth enamel and form a new compound called fluorapatite. This compound is almost insoluble in the acids produced by bacteria in the mouth, so it helps protect teeth from decay.
Application
Sodium fluoride has good antiseptic properties, so it is sometimes added to detergents. It is used for wood processing for the same reason. This salt solution helps fight mold, mildew and insects. Most often a three percent solution is used. It penetrates well into the wood and protects it from rotting. But this product has a drawback, due to which sodium fluoride is rarely used - it is easily washed out of the wood during rains, since this salt is highly soluble in water.
It is also used in the synthesis of certain chemical compounds, in particular freons and insecticides. Fluoride ions stop glycolysis (glucose oxidation), so sodium fluoride is used for biochemical studies.
It is often used in the metallurgical industry for cleaning metal surfaces, as well as during their melting and soldering. The substance is sometimes added to cement, making concrete resistant to acids, and to lubricants to improve heat-resistant qualities.
Its most controversial use is its addition to toothpastes. For teeth, sodium fluoride is a source of fluoride, which is necessary to give bones and teeth strength, and also serves to prevent caries. But with high consumption of this element, negative consequences can occur. Therefore, there is still debate about the use of fluoride as an additive in toothpaste.
Links[edit]
- ↑
Wells, John C. (2008),
Longman Pronunciation Dictionary
(3rd ed.), Longman, pp. 313 and 755, ISBN 9781405881180. According to this source, the alternative pronunciation of the second word /e l ɔːr aɪ d/ and, in UK, as well as /ел¯uərаɪd/. - Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). CRC Press. clause 5.194. ISBN 978-1439855119.
- Lewis, Hazardous Properties of RJ Sax Industrial Materials. 10th ed. Volumes 1–3 New York, NY: John Wiley & Sons Inc., 1999, pp. 3248
- ^ ab Sigma-Aldrich Co. , Sodium fluoride. Retrieved March 17, 2015.
- ^ abc NIOSH Pocket Guide to Chemical Hazards. "#0563" . National Institute of Occupational Safety and Health (NIOSH).
- Martel, B.; Cassidy, K. (2004), Chemical Risk Analysis: A Practical Guide
, Butterworth-Heinemann, p. 363, ISBN 978-1-903996-65-2 - "Top 300 2020". ClinCalc
. Retrieved April 11, 2022. - "Sodium Fluoride - Drug Use Statistics". ClinCalc
. Retrieved April 11, 2022. - ↑
Bourne, volume editor, Geoffrey H. (1986).
Dietary Research and Advice on Health and Disease
. Basel: Karger. item 153. ISBN. 978-3-8055-4341-5. - ↑
Jr, Cornelis Klein, Cornelius S. Hurlbut (1999).
A Manual of Mineralogy: (by James D. Dana)
(21st ed., Rev. Ed.). New York: J. Wiley. ISBN 978-0-471-31266-6. - Selwitz, Robert H; Ishmael, in my midst; Pitts, Nigel B. (January 2007). "Caries". Lancet
.
369
(9555):51–59. DOI: 10.1016/S0140-6736(07)60031-2. PMID 17208642. S2CID 204616785. - Division of Oral Health, National Center for Prevention Services, CDC (1993), Fluoridation Census 1992 (PDF), retrieved 2008-12-29. CS1 maint: multiple names: list of authors (link)
- Haguenauer, D; Welch, W; Shi, B; Tugwell, P; Wells, G. (2000). "Fluoride for the treatment of postmenopausal osteoporosis." Cochrane Database of Systematic Reviews
(4): CD002825. DOI: 10.1002/14651858.CD002825. PMID 11034769. - Westergaard, P; Jorgensen, N. R.; Schwarz, P; Mosekilde, L (March 2008). "Effect of Fluoride Treatment on Bone Mineral Density and Fracture Risk—A Meta-Analysis." Osteoporosis International
.
19
(3): 257–68. DOI: 10.1007/s00198-007-0437-6. PMID 17701094. S2CID 25890845. - Blau, Monte; Ganatra, Ramanik; Bender, Merrill A. (January 1972). "18F-fluoride for bone imaging." Seminars in Nuclear Medicine
.
2
(1): 31–37. DOI: 10.1016/S0001-2998 (72) 80005-9. PMID 5059349. - Ordonez, A.A.; DeMarco, Vice President; Klunk, M. H.; Pokkali, S.; Jain, S.K. (October 2015). "Imaging chronic tuberculous lesions using sodium [18F] fluoride positron emission tomography in mice". Molecular Imaging and Biology
.
17
(5):609–614. DOI: 10.1007/s11307-015-0836-6. PMC 4561601. PMID 25750032. - Grant, F.O.; Fahey, F. H.; Packard, AB; Davis, R. T.; Alavi, A.; Treves, S. T. (December 12, 2007). "18F-Fluoride Skeletal PET: Applying New Technology to an Old Tracer". Journal of Nuclear Medicine
.
49
(1): 68–78. DOI: 10.2967/jnumed.106.037200. PMID 18077529. - Halpern, D.F. (2001), "Sodium fluoride", Encyclopedia of Reagents for Organic Synthesis
, John Wiley & Sons, DOI: 10.1002/047084289X.rs071, ISBN 978-0471936237 - ^ abc Aigueperse, Jean; Mollard, Paul; Devillier, Didier; Chemla, Marius; Faron, Robert; Romano, Rene; Couer, Jean-Pierre (2000). "Inorganic fluorine compounds." Ullman Encyclopedia of Industrial Chemistry
. Weinheim: Wiley-VCH. DOI: 10.1002/14356007.a11_307. - House, James E.; House, Kathleen A. (2015/09/10). Descriptive Inorganic Chemistry. Academic press. item 397. ISBN. 978-0-12-802979-4.
- Metcalfe, Robert L. (2007), "Insect Control", Ullman Encyclopedia of Industrial Chemistry (7th ed.), Wiley, p. 9
- ^ ab Kapp, Robert (2005), "Fluoride", Encyclopedia of Toxicology
,
2
(2nd ed.), Elsevier, pp. 343–346. - Greene Shepherd (2005), "Fluoride", Encyclopedia of Toxicology
,
2
(2nd ed.), Elsevier, pp. 342–343 - NaF Data Safety Data Sheet. hazar.com
- CDC - NIOSH Pocket Guide to Chemical Hazards
- Murray TM, Ste-Marie LG. Prevention and treatment of osteoporosis: consensus statements of the Scientific Advisory Board of the Canadian Osteoporosis Society. 7. Fluoride therapy for osteoporosis. CMAJ
. 1996. 155 (7): 949–54. PMID 8837545. - National Health and Medical Research Council (Australia). A systematic review of the effectiveness and safety of fluoridation
[PDF].
2007. ISBN 1-86496-415-4. Summary: Yeung CA. A systematic review of the effectiveness and safety of fluoridation. Proven dent
. 2008. 9 (2): 39–43. DOI: 10.1038/sj.ebd.6400578. PMID 18584000. - Wells, A.F. (1984), Structural Inorganic Chemistry
, Oxford: Clarendon Press, ISBN 978-0-19-855370-0 - "Chemical and Physical Information", Toxicological Profile of Fluorides, Hydrogen Fluoride, and Fluorine (PDF), Agency for Toxic Substances and Disease Registry (ATDSR), September 2003, page 187, accessed 11/01/2008
- Handbook of Minerals (PDF), mineral data publication, 2005.
Positive effects of fluoride on the body
Fluorine is a fairly important trace element in the human body, without which normal functioning is impossible. An adult should consume 0.03 mg of fluoride per kilogram of body weight per day. The child needs 5 times more.
The functions of fluoride in the body are very diverse. It promotes proper growth and formation of bones, hair and nails, as it stabilizes calcium during the mineralization process. This is especially important during the growth and development of children, as well as during fractures. This element is necessary to maintain immunity. Iron is better absorbed by the body if fluoride is involved in this process.
A deficiency of this element weakens tooth enamel and increases the risk of caries. In this case, children may develop defects in skeletal development. Adults are at risk of developing osteoporosis. This disease is characterized by decreased bone density, which increases bone fragility.
Problems with excess fluoride in the body
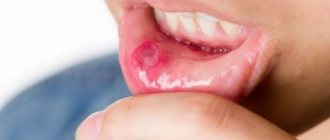
Fluorosis can occur with increased fluoride content in the body. This disease is characterized by a number of irreversible consequences. In the initial stages of the disease, tooth enamel suffers. Spots of different shapes and colors appear on it. The stains are easily diagnosed by a dentist, and with timely treatment they can be easily eliminated. For bleaching, solutions of inorganic acids, hydrogen peroxide solutions, or solutions of other peroxides are often used. After enamel whitening, remineralization is carried out with a solution of calcium gluconate. When treating more severe forms of fluorosis, it is recommended to take calcium gluconate orally until the end of therapy. If erosion of tooth enamel occurs due to fluorosis, then composite materials are used and the shape of the tooth is restored, in much the same way as with filling.
To prevent this disease, you can reduce the intake of fluoride into the body if its concentration in drinking water is high. To do this, they usually replace the water source or simply filter it. You can also remove from your diet foods that contain a lot of fluoride: sea fish, animal oil, spinach. Adding vitamins C and D to your diet, as well as calcium gluconate, may help.
If excess fluoride is observed over a long period (10-20 years), bones begin to suffer. Osteosclerosis occurs, in which, unlike osteoporosis, bone density becomes higher than normal, which leads to a decrease in their elasticity. This can also cause frequent fractures. But there is no need to worry. Such a strong excess of fluoride in the body can only occur in people working in fluoride production without observing safety precautions.
Excerpt describing sodium fluoride
- Very good. - Well, come quickly and drink tea. Together. And Natasha stood on tiptoe and walked out of the room the way dancers do, but smiling the way only happy 15-year-old girls smile. Having met Sonya in the living room, Rostov blushed. He didn't know how to deal with her. Yesterday they kissed in the first minute of the joy of their date, but today they felt that it was impossible to do this; he felt that everyone, his mother and sisters, looked at him questioningly and expected from him how he would behave with her. He kissed her hand and called her you - Sonya. But their eyes, having met, said “you” to each other and kissed tenderly. With her gaze she asked him for forgiveness for the fact that at Natasha’s embassy she dared to remind him of his promise and thanked him for his love. With his gaze he thanked her for the offer of freedom and said that one way or another, he would never stop loving her, because it was impossible not to love her. “How strange it is,” said Vera, choosing a general moment of silence, “that Sonya and Nikolenka now met like strangers.” – Vera’s remark was fair, like all her comments; but like most of her remarks, everyone felt awkward, and not only Sonya, Nikolai and Natasha, but also the old countess, who was afraid of this son’s love for Sonya, which could deprive him of a brilliant party, also blushed like a girl. Denisov, to Rostov’s surprise, in a new uniform, pomaded and perfumed, appeared in the living room as dandy as he was in battle, and as amiable with ladies and gentlemen as Rostov had never expected to see him. Returning to Moscow from the army, Nikolai Rostov was accepted by his family as the best son, hero and beloved Nikolushka; relatives - as a sweet, pleasant and respectful young man; acquaintances - like a handsome hussar lieutenant, a deft dancer and one of the best grooms in Moscow. The Rostovs knew all of Moscow; this year the old count had enough money, because all his estates had been remortgaged, and therefore Nikolushka, having got his own trotter and the most fashionable leggings, special ones that no one else in Moscow had, and boots, the most fashionable, with the most pointed socks and little silver spurs, had a lot of fun. Rostov, returning home, experienced a pleasant feeling after some period of time trying on himself to the old living conditions. It seemed to him that he had matured and grown very much. Despair for failing to pass an exam according to the law of God, borrowing money from Gavrila for a cab driver, secret kisses with Sonya, he remembered all this as childishness, from which he was now immeasurably far away. Now he is a hussar lieutenant in a silver mentic, with a soldier's George, preparing his trotter to run, together with famous hunters, elderly, respectable. He knows a lady on the boulevard whom he goes to see in the evening. He conducted a mazurka at the Arkharovs’ ball, talked about the war with Field Marshal Kamensky, visited an English club, and was on friendly terms with a forty-year-old colonel whom Denisov introduced him to. His passion for the sovereign weakened somewhat in Moscow, since during this time he did not see him. But he often talked about the sovereign, about his love for him, making it felt that he was not telling everything yet, that there was something else in his feelings for the sovereign that could not be understood by everyone; and with all my heart he shared the general feeling of adoration in Moscow at that time for Emperor Alexander Pavlovich, who in Moscow at that time was given the name of an angel in the flesh. During this short stay of Rostov in Moscow, before leaving for the army, he did not become close, but on the contrary, broke up with Sonya. She was very pretty, sweet, and obviously passionately in love with him; but he was in that time of youth when there seems to be so much to do that there is no time to do it, and the young man is afraid to get involved - he values his freedom, which he needs for many other things. When he thought about Sonya during this new stay in Moscow, he said to himself: Eh! there will be many more, many more of these, somewhere, still unknown to me. I’ll still have time to make love when I want, but now there’s no time. In addition, it seemed to him that there was something humiliating for his courage in female society. He went to balls and sororities, pretending that he was doing it against his will. Running, an English club, carousing with Denisov, a trip there - that was another matter: it was befitting of a fine hussar. At the beginning of March, the old Count Ilya Andreich Rostov was preoccupied with arranging a dinner at an English club to receive Prince Bagration. The Count in a dressing gown walked around the hall, giving orders to the club housekeeper and the famous Theoktistus, the senior cook of the English club, about asparagus, fresh cucumbers, strawberries, veal and fish for Prince Bagration's dinner. The Count, from the day the club was founded, was its member and foreman. He was entrusted by the club with arranging a celebration for Bagration, because rarely did anyone know how to organize a feast in such a grand manner, hospitably, especially because rarely did anyone know how and want to contribute their money if they were needed to organize the feast. The cook and housekeeper of the club listened to the count's orders with cheerful faces, because they knew that under no one else could they profit better from a dinner that cost several thousand. - So look, put scallops, scallops in the cake, you know! “So there are three cold ones?...” asked the cook. The Count thought about it. “You can’t do less, three...mayonnaise once,” he said, bending his finger... “So, will you order some large sterlets?” - asked the housekeeper. - What can we do, take it if they don’t give in. Yes, my father, I forgot. After all, we need another entrée for the table. Ah, my fathers! “He grabbed his head. - Who will bring me flowers? - Mitinka! And Mitinka! “Ride off, Mitinka, to the Moscow region,” he turned to the manager who came in at his call, “jump off to the Moscow region and now tell Maximka to dress up the corvée for the gardener. Tell them to drag all the greenhouses here and wrap them in felt. Yes, so that I have two hundred pots here by Friday. Having given more and more different orders, he went out to rest with the countess, but remembered something else he needed, returned himself, brought back the cook and the housekeeper, and again began to give orders. A light, masculine gait and the clanking of spurs were heard at the door, and a handsome, ruddy, with a black mustache, apparently rested and well-groomed from his quiet life in Moscow, entered the young count. - Oh, my brother! “My head is spinning,” the old man said, as if ashamed, smiling in front of his son. - At least you could help! We need more songwriters. I have music, but should I invite the gypsies? Your military brethren love this. “Really, daddy, I think Prince Bagration, when he was preparing for the Battle of Shengraben, bothered less than you do now,” said the son, smiling. The old count pretended to be angry. - Yes, you interpret it, you try it! And the count turned to the cook, who, with an intelligent and respectable face, looked observantly and affectionately at father and son. - What are young people like, eh, Feoktist? - he said, - the old people are laughing at our brother. - Well, Your Excellency, they just want to eat well, but how to assemble and serve everything is not their business. “Well, well,” the count shouted, and cheerfully grabbing his son by both hands, he shouted: “So that’s it, I got you!” Now take the pair of sleighs and go to Bezukhov, and say that the count, they say, Ilya Andreich sent to ask you for fresh strawberries and pineapples. You won't get it from anyone else. It’s not there, so you go in, tell the princesses, and from there, that’s what, go to Razgulay - Ipatka the coachman knows - find Ilyushka the gypsy there, that’s what Count Orlov was dancing with, remember, in a white Cossack, and bring him back here to me. - And bring him here with the gypsies? – Nikolai asked laughing. - Well, well!... At that time, with silent steps, with a businesslike, preoccupied and at the same time Christianly meek look that never left her, Anna Mikhailovna entered the room. Despite the fact that every day Anna Mikhailovna found the count in a dressing gown, every time he was embarrassed in front of her and asked to apologize for his suit. “Nothing, Count, my dear,” she said, meekly closing her eyes. “And I’ll go to Bezukhoy,” she said. “Pierre has arrived, and now we’ll get everything, Count, from his greenhouses.” I needed to see him. He sent me a letter from Boris. Thank God, Borya is now at headquarters. The Count was glad that Anna Mikhailovna took part of his instructions, and ordered her to pawn a small carriage.
Water fluoridation
As mentioned above, fluoride can be used to prevent tooth decay. For this reason, in the middle of the last century, fluoridation of tap water began to be used in some countries. Its essence is reminiscent of chlorination. A small amount of sodium fluoride or another component containing fluoride is added to water to give it certain properties. Today, 2/3 of all water in the United States is fluoridated.
In order for a person to receive the required amount of fluoride, according to the World Health Organization, drinking water should contain 0.5-1.0 mg of fluoride per liter. But ordinary water does not always contain such an amount, so it has to be increased artificially.
Fluoridation of water does not affect its taste or smell in any way. Thanks to this process, the risk of caries is greatly reduced, especially among children. This happens because fluoride kills bacteria that can dissolve tooth enamel and cause tooth decay.
Of course, an increased fluoride content can lead to fluorosis, but, according to authoritative studies, water fluoridation cannot be the cause of the development of this pathology. There are also no other side effects observed with this water. Although recently some low-quality studies have emerged that suggest otherwise. The myth that water fluoridation serves as a method for recycling fluorides, which is a waste product from aluminum plants, was also gaining popularity. But this version has not been confirmed.
Drinking fluoridated water is not recommended only for certain diseases: diabetes, hormonal disorders, arthritis, diseases of the thyroid gland, kidneys and heart.
In any case, it is easy to rid water of the presence of fluoride. Filters operating on the principle of reverse osmosis remove almost all fluoride, and distillation purifies the water completely of it. Household filters can also partially or completely retain fluoride. Passing water through alumina, bone meal or bone char can also remove fluoride from the water. Some fluorides (such as calcium fluoride) are insoluble in water, so a precipitation method can be used to precipitate all the fluoride. Lime is often used for this.
Application in pharmacology
Sodium fluoride is an active ingredient in some medications. As a rule, such tablets are taken as prescribed by a doctor; sometimes therapy requires special monitoring and is accompanied by regular studies of the dynamics of the course. Trade names of fluoride preparations: “Sodium fluoride”, “Natium fluoratum” and “Ossin”. They are prescribed for a lack of fluoride in the body, in particular for osteoporosis.
The drug in the form of dragees and tablets is taken orally. Almost all fluoride is absorbed by the body, regardless of food intake. Typically, this therapy is combined with calcium and magnesium intake at 1-1.5 g per day. This helps bones mineralize more consistently.
But it is dangerous to take the drug in excess of the norm. In this case, excess fluoride may occur, leading to fluorosis. When taking medications containing sodium fluoride, it is necessary to see a dentist to prevent the development of fluorosis.
Interaction
The instructions indicate that calcium salts bind fluorine-containing compounds in the gastrointestinal tract. In addition, drugs based on aluminum or magnesium have a similar effect. Medicines that have enveloping properties also interfere with the absorption of fluoride, so they must be taken separately. Vitamins A and D can enhance the effect of sodium fluoride.
Treatment with sodium fluoride is effective in the fight against tooth decay and osteoporosis, but you should always follow your doctor's recommendations and periodically take blood tests to prevent possible side effects.