From this article you will learn:
- reviews on Stomatofit,
- Features of use for gum inflammation, stomatitis,
- price of the drug in pharmacies for 2022.
Stomatofit is a line of medicines that are prescribed by dentists for the treatment of inflammatory diseases of the gums and oral mucosa. Manufacturer – pharmaceutical (Poland). The drug has 5 release forms. The main forms of release are in the form of a spray, a concentrated solution and a product for lubricating erosions during stomatitis. There are also 2 types of rinses, one of which is intended for children from 6 years old, and the other for teenagers from 14 years old and adults.
The active components of the Stomatofit line of drugs are extracts and essential oils of 7 types of medicinal plants - chamomile, oak bark, sage, arnica, calamus, thyme and peppermint. In addition, the drug “Stomatofit A” additionally contains anesthesin, which anesthetizes erosions due to stomatitis. As for rinses, they additionally contain fluorides, xylitol and thymol, but the concentration of plant extracts themselves in them will be significantly lower (compared to other forms).
Line of drugs Stomatofit –
In this article we will talk about the indications for the use of Stomatofit drugs, the features of their use in the treatment of gum inflammation and stomatitis, positive as well as negative properties that do not always allow their use in some groups of patients. Below we will analyze in order the following forms of release of this drug -
- Stomatophyte (in the form of a solution),
- Stomatofit Expert (spray),
- Stomatofit A (with anesthesin),
- 2 rinses from the Stomatofit Fresh series.
Stomatofit: properties and composition of the drug
Stomatophyte is obtained from plants using ethyl alcohol at a concentration of 70%. The rinse contains oak bark, arnica, chamomile flowers, sage, mint, thyme, calamus rhizomes.
The mouthwash is available in bottles of 50 and 100 ml. The liquid has a characteristic brown tint and is often opalescent. Sediment may form from the herbs, which can be easily removed by shaking.
Mechanism of action
The components of the rinse have astringent, tanning and anti-inflammatory properties. Plant components soften and soothe the inflamed oral mucosa, reduce the activity of leukocytes.
Oak bark and essential oils also have antiseptic properties and reduce the amount of pathogenic microflora and fungi. Clinical studies have shown the effectiveness of chamomile and sage against a wide range of microorganisms, as well as fungi of the genus Candida.
Indications for use
Stomatophyte is used for inflammation of various etiologies and localizations:
- inflammation of the gums in various stages of the process,
- serous or purulent periodontitis,
- inflammatory processes of tongue tissues,
- periodontal disease,
- weakness of blood vessels in the mucous membrane and bleeding gums,
- pathology caused by the introduction of parasites and the consequences of sexually transmitted diseases,
- candidiasis of the mucous membrane.
Contraindications
- Hypersensitivity to any component of the drug.
- Age up to 12 years.
- Pregnancy and lactation period.
- Driving vehicles because the rinse aid contains alcohol.
Application diagram
Before use, shake the rinse aid to remove sediment. Then 5 ml of the product is diluted in 40-50 ml of boiled water at room temperature. Rinse the mouth for at least 30 seconds 3-4 times a day. The drug is prescribed for 10 days, the course can be extended.
Disadvantages of the product
The rinse has a good anti-inflammatory effect, but is not used independently. The drug is prescribed for complex treatment after preliminary sanitation of the oral cavity and removal of tartar.
It is undesirable to use the drug for more than two weeks due to the alcohol it contains. Ethyl alcohol has an irritating effect and dries out the mucous membrane. This leads to a decrease in saliva production and a change in the acidity of the oral cavity. A decrease in acidity leads to dysbiosis and aggravation of the inflammatory process. It is not recommended to use Stomatofit as a prophylactic agent between exacerbations of chronic oral diseases.
Price
- 50 ml of the drug costs 160 rubles,
- volume 100 ml – 200-210 rub.
Stomatofit Expert (spray) –
Stomatofit Expert has exactly the same composition as regular Stomatofit (concentrated rinse solution) - only in the form of a spray.
This makes it possible to use the drug anywhere, for example, at work, in the car or outdoors, and eliminates the need to dilute the concentrated solution of Stomatofit with water. For Stomatofit Expert, the price starts from 300 rubles for a 50 ml spray bottle, which is significantly more expensive compared to a regular concentrated rinse solution. This drug is also not recommended for use by children under 12 years of age, when driving vehicles, or by pregnant and lactating women. The course of application is 10 days, 4 times a day.
Features of Stomatofit A
In addition to herbal components, the product contains the anesthetic Anestezin. Thanks to the anesthetic effect, the product reduces the pain of oral ulcers and at the same time has an anti-inflammatory effect. The drug is produced in small packages of 25 ml, the product is brown in color.
Indications:
- treatment of ulcers during inflammatory processes,
- lubricating damaged mucous membranes when wearing removable dentures.
Contraindications:
- Allergy to the components of the product.
- Age up to 18 years.
- Instructions for use prohibit the use of the mouthwash for pregnant women and nursing mothers.
- When driving a car, use with caution, as this product contains alcohol.
Directions for use: before application, shake the product to remove sediment, lubricate the eroded surface with a swab or cotton swab 3-4 times a day. After treatment, refrain from drinking and eating for 1 hour. The course lasts 5-7 days.
Price – about 150-160 rubles.
The drug "Stomatofit® A" in the complex treatment of inflammatory periodontal diseases
L. N. Maksimovskaya Doctor of Medical Sciences, Professor, Honored Doctor of the Russian Federation, Head of the Department of General Dentistry of the Faculty of Advanced Dentistry of the Moscow State Medical University
T. D. Chirkova Ph.D., Associate Professor, Department of Faculty Therapeutic Dentistry, Moscow State Medical University
P. V. Kuprin Assistant, Department of General Dentistry, Department of General Practice Dentistry, FPDO MGMSU
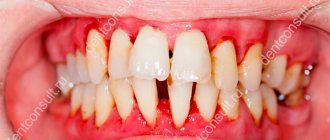
Lack of adequate oral hygiene, combined with internal and external unfavorable factors, often leads to the development of inflammatory diseases of the periodontium (gingivitis, periodontitis) and the oral mucosa (stomatitis).
Pain in the gums, discomfort in the mouth, bad breath, bleeding gums both when brushing teeth and for no apparent reason are the main symptoms of an inflammatory process in the gums. Complex treatment indicated for this pathology includes many different components: professional oral hygiene, surgical methods, drug therapy, including various rinses that have antiseptic, antibacterial and anti-inflammatory effects. Essential oils and other components contained in medicinal plants that are part of the drug "Stomatofit® A" have antibacterial, antiseptic, and anti-inflammatory effects. They reduce inflammation of the oral mucosa and have a local anesthetic, softening and deodorizing effect. Benzocaine has a local anesthetic effect. Thickeners in the composition of “Stomatofit® A” help to retain the drug on the affected area, which ensures a longer effect.
CHAMOMILE FLOWERS (FLORES CHAMOMILLAE)
Contain compounds that have anti-inflammatory and antibacterial effects: essential oil containing bisabolol and bisabolol oxides, chamazulene, farnesene; flavonoids (apigenin, luteolin). Pharmacological and clinical studies have confirmed that chamomile extracts are growth inhibitors of Staphylococcus and Streptococcus strains and have a mycostatic effect.
OAK BARK (CORTEX QUERCUS)
The tannins in oak bark have a strong astringent effect. They form insoluble compounds with proteins, thereby protecting the surface of the oral mucosa and at the same time having a detrimental effect on microorganisms. Extracts from oak bark have an anti-inflammatory effect and reduce bleeding gums.
SAGE LEAVES (FOLIUM SALVIAE OFFICINALIS)
They contain essential oil with a strong antiseptic, bactericidal, mycostatic (mainly Candida albicans), antiviral effect, as well as a tannin with an astringent and anti-inflammatory effect. This composition makes sage leaves effective against inflammatory and infectious diseases of the oral mucosa.
ARNICA GRASS (HERBA ARNICAE)
It has an anti-edematous effect, promotes the growth of granulation tissue, and improves microcirculation processes. Essential oil and phenolcarboxylic acids determine the antiseptic and bactericidal effect.
Calamus rhizome (ACORUS CALAMIS L.)
Calamus extract is used externally for inflammation of the mouth and throat as a rinse and for some dermatoses as an antibacterial, antipruritic and anti-inflammatory bath agent (Oarowski, 1980).
PEPPERMINT (MENTHA PIPERITA L.)
According to the monographs of Commission E and ESCOP, peppermint leaf is a raw material that has an antispasmodic effect, enhances the secretion of digestive juices (including bile), and has antibacterial, analgesic and antiseptic effects.
Peppermint leaf is a typical oilseed raw material, essential oil is its main component. Contained in leaves in an amount of 0.5-4.0% (in some cultivated species up to 3.5%).
The main component of the oil is menthol (more than 50%). In addition, peppermint essential oil contains esters: acetate and valerian, menthone, phellandrene, pinene, cineol, mentofuran, piperitone, jasmon.
The components of peppermint leaf are also tannins 6-12%, flavonoids: luteolin, apigenin, diosmetin, bitterness, phenolic acids (Mills, Bone, 2000; Kohlmnzer, 2000). The component with the described activity is considered to be an essential oil that has an antiseptic effect, reduces sensitivity and at the same time stimulates nerve endings; tannins and flavonoids also participate in these therapeutic effects.
THYME HERB (THYMUS VULGARIS L.)
Thyme herb has a positive monograph of Commission E, ESCOP, monograph of the European Pharmacopoeia, DAB10, PF V. The scope of application, according to the monograph of Commission E, is symptoms of bronchial catarrh and whooping cough, catarrh of the upper respiratory tract (Komisja E., 1990). The ESCOP monograph expands the indications: inflammation of the oral cavity and bad breath (halithosis) (ESCOP, 1996).
Numerous pharmacological studies confirm the antispasmodic and expectorant, as well as antibacterial, antifungal and disinfectant effects of this raw material. Thyme oil has a strong antibacterial effect against both gram-negative and gram-positive strains. It also acts on fungi and yeasts, such as Candida albicans. This activity is due to the action of thymol and carvacrol.
The drug "Stomatofit® A" is a liquid extract from a mixture of medicinal plant materials (chamomile flowers, oak bark, sage leaves, arnica herb, calamus rhizomes, peppermint herb, common thyme herb) and anesthesin; has anti-inflammatory, astringent, antiseptic and local anesthetic effects. All of these components are well known: their pharmacological properties and clinical effectiveness have been proven by research by domestic and foreign scientists. The components of the drug are widely used in medicine, both independently and as part of various drugs.
25 g of the drug contains:
- Active components
Liquid extract from a mixture of raw materials (0.65:1.0) - 12,500 mg, containing: chamomile flowers (Matricaria chamomilla L) 1625 mg, oak bark (Quercus robur L) 1625 mg, sage leaves (Salvia officinalis L.) 1625 mg, arnica herb (Arnica Montana L.) 812 mg, calamus rhizomes (Acorus calamis L.) 812 mg, peppermint (Mentha piperita L) 812 mg, common thyme herb (Thymus vulgaris L.) 812 mg.
- Anestezin (benzocaine) 500 mg.
- Excipients
Sodium tetroborate, glycerin (glycerol), methylcellulose, ethanol 96%, purified water.
The drug is standardized for the content of essential oils (not less than 0.075%) and anesthesin (1.8-2.2%), as the main groups of compounds that determine therapeutic activity.
Essential oils and other components contained in these medicinal plants that are part of the drug "Stomatofit® A" have antibacterial, antiseptic, and anti-inflammatory effects. They reduce inflammation of the oral mucosa and have a local anesthetic, softening and deodorizing effect.
Benzocaine has a local anesthetic effect. Thickeners in the composition of “Stomatofit® A” help to retain the drug on the affected area, which ensures a longer effect.
PURPOSE OF THE STUDY
Evaluation of the effectiveness and safety of the use of the herbal preparation “Stomatofit® A” when used in the complex treatment of inflammatory diseases of the oral cavity.
MATERIALS AND METHODS OF RESEARCH
An open comparative, randomized study of the drug "Stomatofit® A" (manufacturer - joint stock company "Fitofarm Klenka", Poland) when used in the complex treatment of inflammatory diseases of the oral cavity in comparison with the effectiveness of the drug "Stomatofit®" in the complex treatment of inflammatory diseases of the oral cavity ( manufacturer - joint stock company "Fitofarm Klenka", Poland).
In accordance with the inclusion and exclusion criteria of the clinical trial protocol, the study included 120 patients who, according to the randomization plan set out in the protocol, were divided into the main (60 patients) and control groups (60 patients). The distribution of patients is presented in Table No. 1.
Table No. 1. Distribution of patients included in the study by gender and age
| From 18 to 33 years old | From 33 to 50 years | From 50 to 65 years | Total | ||
| Main group (1) | Men | 17 | 5 | 2 | 24 |
| Women | 17 | 12 | 7 | 36 | |
| Control group (1) | Men | 14 | 6 | 1 | 21 |
| Women | 19 | 13 | 7 | 39 | |
Under our supervision there were 120 patients diagnosed with: chronic catarrhal gingivitis in the acute stage (CHG) - 35 (main group); mild chronic generalized periodontitis in the acute stage (CHP LS) - 16 (main group); chronic generalized periodontitis of moderate severity (CGPS) - 9 (main group).
The control group included 36 patients with exacerbation of chronic catarrhal gingivitis, 16 patients with mild chronic generalized periodontitis and 8 patients with moderate chronic generalized periodontitis (CGPS), also in the acute stage (Fig. 1).
Rice. 1. Distribution of patients in the main and control groups by nosological forms
In accordance with the criteria for early termination of participation in the study and early termination of the study, approved in the clinical trial protocol, none of the patients in the main and control groups were prematurely excluded. Concomitant diseases of patients in the main and control groups are presented in Table No. 2.
Rice. 2. Final integration assessment of the effectiveness of the studied drugs
Pharmacotherapy for concomitant diseases of patients in the main and control groups did not change throughout the study and did not include drugs that affect the course of the clinical trial. The remaining patients of the main and control groups were practically healthy. After signing informed consent to participate in the study, patients of the main and control groups underwent a screening examination, which included:
- assessment of compliance with inclusion/exclusion criteria;
- registration of demographic data (date of birth, gender);
- collecting a medical history, including past illnesses and surgeries;
- objective examination;
- questioning;
- registration of medication prescriptions in the three-month period preceding the study;
Physical examination:
- according to indications;
- X-ray methods;
- ECG, blood pressure, pulse;
- clinical and biochemical blood test (total protein, ALT, AST, glucose, urea, creatinine).
After the examination, patients received medications and began taking them.
Table No. 2. Concomitant diseases of patients of the main and control groups
| Nosology | Group of subjects (number of patients) | Control group (number of patients) |
| Gastrointestinal diseases | 9 | 9 |
| Arterial hypertension 1-2 degrees. | 7 | 7 |
PRESCRIPTION SCHEME OF STUDY DRUGS
Patients of both groups with chronic catarrhal gingivitis in the acute stage and periodontitis of mild and moderate severity in the acute stage received adequate treatment (removal of supra- and subgingival deposits, professional oral hygiene, curettage), corresponding to the initial stage of treatment of inflammatory periodontal diseases.
Patients in the main group were prescribed the drug "Stomatofit® A". The affected areas were smeared with the drug, previously applied to a cotton swab, four times a day. Immediately after using the drug, patients refrained from eating and rinsing the mouth. The duration of the course is 7 days.
Patients in the control group were prescribed the drug "Stomatofit®" in the form of mouth rinses with a 15% aqueous solution of the drug four times a day (7.5 ml of the drug dissolved in 1/4 cup of boiled water). The duration of the course is 7 days. Control medical examinations were carried out on the third, fifth and seventh days of the study.
During the study, patients were prohibited from using medications that could affect the study results:
- use of non-steroidal anti-inflammatory drugs;
- use of GCS for systemic therapy;
- use of antibacterial agents;
- rinsing the mouth with chlorhexidine;
- rinsing the mouth with antiseptic and/or astringent solutions.
The effectiveness of therapy was assessed using the following parameters:
- Assessment of the level of oral hygiene based on the dynamics of the OHI-S oral hygiene index.
- Assessment of the dynamics of the inflammatory process in the gums using the PMA index.
- Assessment of gum bleeding using the PBI (papilla bleeding index): Muehlemann, Son (1971).
- Assessment of the severity of pain using VAS (visual analogue scale) on a ten-point system.
- Integral efficiency assessment.
The subjective assessment of the results of treatment by the patient and the assessment of the results of treatment by the doctor and compliance were taken into account.
Data from medical examinations and laboratory test results were entered into individual patient records and subjected to statistical processing.
Assessment of tolerability and safety of study drugs
Tolerability and safety assessments were based on subjective complaints and objective data obtained during the study. The dynamics of clinical and biochemical blood test parameters (total protein, ALT, AST, glucose, urea, creatinine), frequency of occurrence and nature of side effects were taken into account.
Tolerability of the drug was assessed using objective and subjective data (Table No. 3) and in points.
Table No. 3. Tolerability of the drug
| good | No adverse reactions were identified. |
| Satisfactory | Minor adverse reactions are observed that do not require discontinuation of the drug. |
| Unsatisfactory | Undesirable side reactions occur that have a significant negative impact on the patient’s condition, requiring discontinuation of the drug and additional medical measures. |
| The safety and tolerability of the drugs were also assessed in points on the following scale: 0 - the drug was discontinued due to side effects; 1 point - side effects that do not require discontinuation of the drug; 2 points - the drug is well tolerated. | |
RESEARCH RESULTS AND DISCUSSION
The use of the drug "Stomatofit® A" in the complex treatment of inflammatory periodontal diseases did not reveal any adverse reactions that had a significant negative impact on the patient's condition, requiring discontinuation of the drug and additional medical measures. The safety of “Stomatofit® A” is confirmed by laboratory data (Table No. and the positive dynamics of clinical symptoms.
The safety of “Stomatofit® A” is confirmed by laboratory data (Table No. and the positive dynamics of clinical symptoms.
The effectiveness of the drug "Stomatofit® A" is confirmed by analyzing the dynamics of the main clinical symptoms in patients of the main group. Prescribing "Stomatofit® A" to patients in combination with professional hygiene significantly increased the level of oral hygiene (Table No. 4). Thus, the UIG from 2.76 ± 0.18 at the first visit decreased significantly by the second visit to 0.29 ± 0.05; by the end of treatment in patients of the main group, the level of oral hygiene became satisfactory (UIG - 0). In the control group, in which patients used rinsing with Stomatofit®, a significant increase in the level of oral hygiene was also observed: IIG decreased from 2.42 ± 0.18 to 0.075 ± 0.03 at the end of the treatment course.
Table No. 4. Dynamics of the oral hygiene indicator in patients of the main and control groups during therapy (OHI-S oral hygiene index)
| Groups | Stomatofit® A (main group) | Stomatofit® (control group) | ||||
| Index | IZN | FROM TO | UIG | IZN | FROM TO | UIG |
| Visit 1 | 1,6 ± 0,09 | 1,2 ± 0,08 | 2,76 ± 0,18 | 1,25 ± 0,09 | 1,12 ± 005 | 2,42 ± 0,18 |
| Visit 2 | *0,26 ± 0,03 | *0,06 ± 0,02 | 0,29 ± 0,05 | *0,43 ± 0,04 | *0,09 ± 0,02 | *0,53 ± 0,05 |
| Visit 3 | *0 | *0 | *0 | *0,14 ± 0,006 | *0,008 ± 0,009 | *0,15 ± 0,03 |
| Visit 4 | *0 | *0 | *0 | *0,07 ± 0,03 | *0 | *0,075 ± 0,03 |
* p < 0.05 - the probability of differences is indicated in comparison with the beginning of the study
It should be noted that no significant difference in the results of the influence of the use of “Stomatofit® A” and “Stomatofit®” was identified. The dynamics of the results are similar (p > 0.05).
The use of "Stomatofit® A" in combination with professional oral hygiene showed a decrease in gum bleeding from 3.1 ± 0.09 to 2.2 ± 0.09 by the second visit (Table No. 5), by the third - to 1.33 ± 0.09, by the fourth visit, gum bleeding was practically absent in 76% (23 patients). In the control group, the use of the comparison drug also revealed a positive trend in reducing bleeding. By the end of treatment, bleeding persisted in the form of single pinpoint bleeding. A steady decrease in signs of inflammation was confirmed by the positive dynamics of the PMA index in both groups; a more intense anti-inflammatory reaction was observed in the main group (Table No. 6).
Table No. 5. Assessment of bleeding gums using the PBI bleeding index (papilla bleeding index), Muleman, Son (1971)
| Groups | Stomatofit® A (main group) | Stomatofit® (control group) |
| Index | Bleeding PBI | Bleeding RBI |
| Visit 1 | 3,1 ± 0,09 | 2,75 ± 0,13 |
| Visit 2 | *2,2 ± 0,09 | *1,91 ± 0,009 |
| Visit 3 | *1,33 ± 0,09 | *1,25 ± 0,13 |
| Visit 4 | *0,83 ± 0,04 | *0,75 ± 0,09 |
* p < 0.05 - the probability of differences is indicated in comparison with the beginning of the study
Table No. 6. Inflammation assessment using the PMA index
| Groups | Stomatofit® A (main group) | Stomatofit® (control group) |
| Index | РМА (%)I | RMA (%) |
| Visit 1 | 3,1 ± 0,09 | 43,25 ± 1,8 |
| Visit 2 | *29,7 ± 1,9 | *27,3 ± 1,9 |
| Visit 3 | *16,3 ± 1,36 | *16,5 ± 1,95 |
| Visit 4 | *2,9 ± 1,36 | *8,9 ± 1,36 |
* p < 0.05 - the probability of differences is indicated in comparison with the beginning of the study
By the end of the course of treatment, inflammation of the interdental papillae remained in 13% (4 patients) in the main group and in 16% (5 patients) in the control group, respectively, RMA decreased from 55.3 ± 2.7 to 2.9 ± 1.36 in the main group, in the control group - from 43.25 ± 1.8 to 8.9 ± 1.36.
Since exacerbation of inflammatory periodontal diseases is accompanied by pain of varying severity, great importance was attached to the analysis of the analgesic effect of “Stomatofit® A” (Table No. 7).
Table No. 7. Assessment of the severity of pain using VAS (visual analogue scale) on a ten-point system
| Groups | Stomatofit® A (main group) | Stomatofit® (control group) |
| Index | YOUR | YOUR |
| Visit 1 | 7,1 ± 0,22 | 5,87 ± 0,27 |
| Visit 1 | *4,7 ± 0,18 | *4,5 ± 0,22 |
| Visit 1 | *2,9 ± 0,18 | *3,3 ± 0,27 |
| Visit 1 | *1,86 ± 0,13 | *3,1 ± 0,18 |
* p < 0.05 - the probability of differences is indicated in comparison with the beginning of the study
Comparison of changes in the severity of pain according to VAS in the main and control groups revealed significant differences in the analgesic effect of the drug “Stomatofit® A”, this is especially noticeable at the end of treatment (visits 3 and 4). Thus, in patients of the main group, by the third visit, pain decreased from 7.1 ± 0.22 to 2.9 ± 0.18 points and decreased significantly by the end of the course of treatment (1.86 ± 0.13) (Table No. 7) .
The use of the comparison drug "Stomatofit®" showed a moderate analgesic effect, the pain syndrome decreased from 5.87 ± 0.27 to 4.5 ± 0.22 by the second visit, to 3.3 ± 0.27 by the third and practically remained the same the same level by the fourth visit - 3.1 ± 0.18.
The inclusion of the anesthetic component anesthesin in the composition of the drug significantly increased the analgesic effect of the drug compared to Stomatofit®, as can be seen from the data in Table No. 7.
Analysis of the dynamics of biochemical and clinical blood tests did not reveal any significant changes. There were no significant deviations from the norm in the blood (Table No. 8).
Table No. 8. Data from laboratory tests in patients of the main and control groups
| Indicators studied | Stomatofit® A | Stomatofit® | ||
| Before treatment | After treatment | Before treatment | After treatment | |
| Hemoglobin, g/l M | 128,4 ± 11,2 | 129,5 ± 8,6 | 129,2 ± 6,3 | 130,3 ± 5,7 |
| Hemoglobin, g/l F | 116,3 ± 13,6 | 118,4 ± 9,4 | 112,7 ± 11,3 | 113,2 ± 11,3 |
| Red blood cells | 5,6 ± 0,4 | 4,6 ± 0,3 | 4,3 ± 0,5 | 4,2 ± 0,3 |
| Color indicator | 1,05 ± 0,09 | 1,1 ± 0,08 | 1,08 ± 0,06 | 0,95 ± 0,09 |
| Leukocytes x 10 in 9 st./l | 5,6 ± 0,4 | 5,9 ± 0,5 | 5,7 ± 0,3 | 5,8 ± 0,6 |
| Lymphocytes, % | 24,7 ± 2,1 | 27,9 ± 1,8 | 25,3 ± 2,2 | 26,1 ± 1,8 |
| Monocytes, % | 5,3 ± 1,6 | 5,6 ± 1,6 | 5,5 ± 1,8 | 5,6 ± 2,1 |
| Platelets x 10 in 9 tbsp/l | 280 ± 33,2 | 245 ± 44,2 | 261 ± 33,7 | 255 ± 28,1 |
| ESR, mm/hour | 10,5 ± 1,3 | 8,9 ± 1,7 | 10,3 ± 1,4 | 8,9 ± 1,2 |
| Total protein, g/l | 79,5 ± 2,1 | 78,2 ± 1,8 | 71,3 ± 1,4 | 76,2 ± 1,7 |
| Creatinine, µmol/l | 86,3 ± 3,1 | 84,2 ± 4,9 | 89,7 ± 4,9 | 87,5 ± 5,4 |
| Urea, mol/l | 5,6 ± 0,5 | 5,7 ± 0,3 | 5,91 ± 0,5 | 5,6 ± 0,6 |
| Glucose, mol/l | 4,5 ± 0,2 | 4,7 ± 0,3 | 4,9 ± 0,4 | 4,9 ± 0,2 |
| AlAT, EU/L | 24,5 ± 0,4 | 24,3 ± 0,7 | 29,1 ± 0,5 | 25,8 ± 0,6 |
| ASAT , EU/L | 33,6 ± 0,9 | 32,5 ± 0,5 | 34,3 ± 1,3 | 33,1 ± 0,8 |
During observation of the patients of the main group, not a single adverse event was registered, which, along with the absence of negative dynamics in laboratory and clinical studies, allows us to recognize the tolerability of “Stomatofit® A” as good. The absence of side effects of Stomatofit® A is confirmed by the assessment of the tolerability of the studied drugs by the doctor and patients (Fig. 2). The drug "Stomatofit® A" demonstrated high anti-inflammatory activity and a pronounced analgesic effect, which was determined by the content of anesthesin in the composition. "Stomatofit® A" is easy to use, the patient can use it independently in accordance with the recommendations of the attending physician.
CONCLUSIONS
The use of the drug “Stomatofit® A”, which has antibacterial activity, in the complex treatment of inflammatory periodontal diseases contributes to the complete normalization of the hygienic state of the oral cavity (UIG, visit 1 - 2.76 ± 0.18, GI at the end of treatment - 0).
The use of "Stomatofit® A" leads to a significant decrease in the PMA index (from 55.3 ± 2.7% to 2.9 ± 1.36%), which is due to the anti-inflammatory effect of the drug.
Therapy with “Stomatofit® A” in combination with oral hygiene helps to significantly reduce bleeding gums, reducing the bleeding index from 3.1 ± 0.09 to 0.83 ± 0.04.
“Stomatofit® A” has a pronounced analgesic effect and helps reduce the severity of pain (from 7.1 ± 0.22 to 1.86 ± 0.13 according to VAS).
An analysis of the dynamics of the main clinical symptoms of inflammatory periodontal diseases showed that the therapeutic effectiveness of Stomatofit® A is comparable to the effectiveness of the reference drug Stomatofit®. At the same time, the analgesic effect of “Stomatofit® A” significantly (p < 0.05) exceeds the effect of the comparison drug.
The absence of undesirable side effects has proven the safety of using “Stomatofit® A”, taking into account the individual tolerability of the drug components. The positive dynamics of clinical symptoms, confirmed by the results of the index assessment, proved the high effectiveness of the drug.
CONCLUSION
Based on the results of the studies, it should be concluded that the drug “Stomatofit® A” has a pronounced therapeutic effect when used in the complex treatment and prevention of inflammatory periodontal diseases (Fig. 2). An excellent effect of using “Stomatofit® A” was noted by 53% (32 patients), a good effect by 47% (28 patients). 47% (28 patients) noted the treatment of inflammatory periodontal diseases with “Stomatofit®” as excellent, a good effect was observed in 43% (26 patients), a satisfactory effect was noted by 10% (6 patients) (Fig. 2).
“Stomatofit® A” is easy to use, easily applied to the gums and is well appreciated by patients, has a pleasant herbaceous taste, and no adverse reactions have been observed in any case.
The drug "Stomatofit®" can be recommended for use in the dosage recommended by the manufacturer in the complex therapy of inflammatory periodontal diseases.
Stomatofit Fresh
The product is additionally used after dental hygiene. The plant components of the drug prevent the development of inflammation and slow down the growth of pathogenic bacteria, and tanning agents promote the healing of minor injuries. In addition, the rinse does not contain alcohol or artificial antiseptics, so it can be used continuously as an additional hygiene product.
Composition and properties of components:
- Sodium fluoride slows down the development of carious lesions and helps increase the thickness of the enamel; in addition, this component has antiseptic properties.
- Essential oils, especially sage and thyme oils, have anti-inflammatory and emollient properties. Peppermint oil and menthol mask the unpleasant odor.
- Xylitol allows you to balance the content of acids and alkalis in the oral cavity, which reduces the rate of reproduction of pathogenic bacteria and the likelihood of dental caries.
Directions for use: rinse your mouth after brushing for 30-60 seconds. After rinsing, it is advisable not to drink for about an hour. The greatest benefit will come from simultaneous use of pastes with added calcium. The combination of sodium fluoride and calcium-containing paste makes it possible to double the anti-caries effect of the mouthwash.
The product is contraindicated for children under 14 years of age.
The price of the rinse aid is 130 rubles.
Stomatofit Fresh for children
The drug is intended for use after the age of 6 years. The product does not contain dyes or other components that Stomatofit Fresh for adults contains as stabilizers.
The composition of the product is similar to Stomatofit Fresh:
- Sodium fluoride 227 ppm protects tooth enamel from caries and helps reduce the number of pathogenic microorganisms.
- Xylitol helps bring the pH of the oral cavity back to normal, which also helps slow down the growth of bacteria and reduces the amount of plaque.
- Essential oils have an anti-inflammatory effect, and menthol freshens breath.
Directions for use: rinse the mouth 1-2 times a day after brushing your teeth with calcium-containing pastes. Using a measuring cap, measure out 5-10 ml of the product and rinse the mouth for 1 minute. After the procedure, do not drink or eat for 1 hour. In addition, it is not advisable to rinse your mouth with water after using Stomatofit Fresh.
Reviews from experts about the children's mouthwash from the Stomatofit line are positive. During use, make sure that the child does not swallow the rinse aid. The price of the product is about 125 rubles.