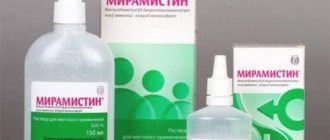
Tantum Verde spray for topical use 0.15% 30ml No. 1
Name
Tantum Verde spray for topical approx. 0.15% in bottle 30 ml in pack No. 1
Description
Colorless transparent liquid with a characteristic mint odor.
Main active ingredient
Benzydamine
Release form
Spray
pharmachologic effect
Pharmacodynamics
Benzydamine is a non-steroidal anti-inflammatory drug. Has anti-inflammatory and local analgesic effect. The mechanism of action of the drug is associated with its suppressive activity of pro-inflammatory cytokines, since it inhibits the synthesis of TNF? and, to a lesser extent, IL-1? and MCP-1, and inhibition of prostaglandin synthesis. Clinical studies have shown that benzydamine is effective in the treatment of localized inflammatory processes in the mouth and throat.
Pharmacokinetics
When applied topically, the drug is well absorbed through the mucous membranes and penetrates into inflamed tissues, reaching effective concentrations due to its ability to penetrate the superficial epithelial layer. Excretion of the drug occurs mainly by the kidneys and through the intestines in the form of metabolites or conjugation products. Dosage forms for topical use do not have a systemic effect.
Indications for use
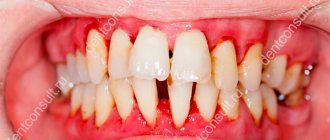
Symptomatic treatment of irritations and inflammatory conditions, including those associated with pain in the mouth and throat (eg, gingivitis, stomatitis, pharyngitis), as well as after conservative treatment or tooth extraction.
Precautionary measures
The use of this drug, especially with long-term therapy, may cause a sensitivity reaction. In this case, treatment should be interrupted and a doctor should be consulted to determine acceptable therapy. In a limited number of patients, the oropharyngeal form of an ulcer may be a sign of more serious pathologies. If symptoms persist beyond 3 days, the patient should consult their doctor or dentist, as appropriate. The use of benzydamine is not recommended in case of hypersensitivity to salicylic acid or other NSAIDs (non-steroidal anti-inflammatory drugs). The drug should be prescribed with caution to patients with a history of bronchial asthma, since bronchospasms may occur in patients in this group. Tantum Verde contains methyl parahydroxybenzoate, which can cause allergic reactions (including delayed ones). For patients involved in sports: the use of drugs containing ethyl alcohol may test positive for doping according to the blood alcohol limits set by some sports federations.
Interaction with other drugs
No clinically significant interaction of Tantum® Verde with other drugs has been established.
Contraindications
Hypersensitivity to any of the components of the drug. Pregnancy and breastfeeding There are no adequate data available on the use of benzydamine during pregnancy and breastfeeding. The ability of this drug to pass into breast milk has not been studied. Data from animal studies of the drug are insufficient to draw any conclusions about the effect of this drug during pregnancy and breastfeeding. The potential risk to humans cannot be assessed. Tantum Verde should not be used during pregnancy and breastfeeding.
Compound
Composition per 100 ml of spray solution for topical use Active ingredient - benzydamine hydrochloride - 0.15 g (which corresponds to 0.045 g in one 30 ml bottle); Excipients: ethanol 96%, glycerol, methyl parahydroxybenzoate, menthol flavor (flavor), saccharin, sodium bicarbonate, polysorbate 20, purified water.
Directions for use and doses
2-6 times a day (each spray is equivalent to 0.17 ml of solution). Adults and adolescents over 12 years of age: 4-8 sprays 2-6 times daily. Children (ages 6-12 years): 4 sprays 2-6 times daily. Children (4-6 years): 1 spray for every 4 kg of child's body weight, maximum dose 4 sprays 2-6 times a day. Do not exceed the prescribed dose. Course of treatment: for inflammatory diseases of the oral cavity and ENT organs (of various etiologies): 3-5 days; for odonto-dental pathology: 3-5 days When using the drug for a long time, consultation with a doctor is necessary. How to use the medication bottle Lift the nozzle of the nebulizer; Insert the nozzle into your mouth and point it towards the inflamed area or throat, in case of throat diseases. Press firmly with your finger on the knurled surface of the button.
Overdose
Very rarely, overdose symptoms such as excitability, convulsions, sweating, ataxia, tremor and vomiting have been reported in children after oral administration of benzydamine in doses that are approximately 300 times higher than recommended. In case of acute overdose, only symptomatic treatment is possible; The stomach should be cleared by inducing vomiting or gastric lavage, and the patient should be under medical supervision with supportive care and adequate hydration.
Side effect
The table below shows side effects classified by organ or organ system according to MedDRA. The frequency categories of side effects are defined as follows: very common (? 1/10); frequent (from ? 1/100 to
Storage conditions
At a temperature not exceeding 25°C in a place protected from light. Keep out of the reach of children!