Dental stainless steel alloy - dental steel
Steel is the most common alloy in the world.
Its properties are well known. And due to alloying agents, it can be given any desired properties. Dental steel is very cheap.
Among the disadvantages: steel is heavy (density about 8 g/cm3) and chemically active. May cause allergies, galvanosis.
Stainless steel in orthopedic dentistry - grades:
STEEL GRADE 1X18H9T (EYA-1)
Dental alloy for crowns COMPOSITION :
1.1% carbon; 9% nickel;18% chromium; 2% manganese, 0.35% titanium, 1.0% silicon, the rest is iron.
Used for fixed prostheses: individual crowns, cast teeth, facets.
STEEL GRADE 20Х18Н9Т
COMPOSITION: 0.20% carbon, 9% nickel, 18% chromium, 2.0% manganese, 1.0% titanium, 1.0% silicon, the rest is iron.
The following are produced from this type of steel in the factory:
· standard sleeves used for the production of stamped crowns;
· clasp blanks (for ChSPP)
· elastic metal matrices for filling, as well as separation strips
· STEEL for dentistry GRADE 25Х18Н102С
COMPOSITION: 0.25% carbon, 10.0% nickel, 18.0% chromium, 2.0% manganese, 1.8% silicon, the rest is iron.
APPLICATION: manufactured in the factory:
· teeth (lateral upper and lower) for stamped-soldered bridges;
· frames for metal-plastic bridges, for cladding;
· orthodontic wire with a diameter from 0.6 to 2.0 mm (step 0.2 mm)
Silver solder is used as solder for non-precious alloys
Contains silver - 37%, copper - 50%, Manganese - 8-9%, Zinc - 5-6%
Source
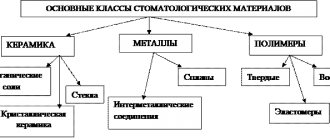
Metals and alloys used in orthopedic dentistry
Properties of metals
In the solid state, metals have a clearly defined crystalline structure; under a microscope, a clear structure can be seen (if the metal is thoroughly polished). The atoms form the crystal lattice of the metal.
The most common crystal lattices are:
- Body-centered cubic (chromium, molybdenum, vanadium).
- Cubic face-centric (nickel, copper, lead).
- Hexagonal (titanium, zinc).
Technological properties
- Fluidity is the ability of metals to fill a mold. An increase in the melting temperature of metals sharply increases its fluidity; when the temperature increases by more than 100, 150 above the melting temperature, the absorption of gases increases, and gas shells may appear.
- Liquation is the appearance of heterogeneity during solidification of the alloy. Deteriorates viscosity and plasticity. Reduces the corrosion resistance of metals.
Types of liquation:
- Alloy cooling ratea
- State diagram type
- Difference in densities of alloy components
- Malleability is a property of metals and alloys that makes it possible to subject them to forging and other types of processing (rolling, drawing, stamping). Malleability is characterized by ductility - the ability of a metal to undergo deformation under pressure without fracture.
- Weldability is the property of metals to produce, with the established welding technology, a connection that meets the requirements determined by the design and operating conditions.
- Hardenability.
Chemical properties
- Corrosion resistance
- Solubility
- Oxidability
- Heat resistance
Physical properties
- Color
- Density
- Melting T
- Thermal conductivity
- Expansion and contraction during heating and cooling, during phase transformations
Mechanical properties
- Hardness
- Elasticity
- Viscosity
- Plastic
- Fragility
Types of alloys
- Mechanical mixture – low-melting alloys based on tin, lead and bismuth.
- Solid solutions – chromium-nickel alloys.
- Chemical compounds – titanium nickelide, titanium nitride, carbides, etc.
- An alloy in the form of a mechanical mixture occurs when metals have complete mutual insolubility and do not form chemical compounds. The atoms of each metal form separate crystal lattices, and the solidified alloy consists of a mechanical mixture of grains of each component (lead and antimony, cadmium and bismuth).
- Solid solutions are formed by elements that are mutually soluble in liquid and solid states. A solid solution is a homogeneous crystalline body in which atoms of the dissolved substance (nickel-chromium, nickel-copper) enter the lattice of the base metal (solvent).
- Chemical compounds. In alloys, chemical compounds are formed that have a variable composition. The crystal lattice differs from the lattices of the formed elements, and significantly changes all properties (iron and chromium carbides, Fe3C and Cr3C2, Mg2S)
Noble alloys
- Gold-based alloys.
- Silver-based alloys.
- Palladium-based alloys.
Gold (Au).
Gold has a yellow color and a bright metallic luster; it is found in its native state and in the form of various impurities.
- Density 19.32 g/cm3
- Melting temperature 1063.5 0С
- Brinell hardness 18.5 kgf/mm2
- Solidification shrinkage 1.2%
Pure gold is a very soft metal and is highly resistant to corrosion. It is not affected by acids and alkalis (except for aqua regia). High anti-corrosion properties are used when separating pure gold from various alloys by refining, which separates the metal from the gold.
| Compositions of gold-based alloys | |||
| Component | 900 | 750 | Solder 750 |
| Gold | 90 | 75 | 75 |
| Silver | 4 | 8 | 3 |
| Copper | 6 | 8 | 10 |
| Platinum | – | 9 | – |
| Cadmium | – | – | 12 |
| Impurities | – | no more than 0.3% | no more than 0.3% |
To lower the melting point, cadmium is added to the solder.
Silver (Ag).
Silver is found in nature in the form of nuggets, as well as in chemical compounds with sulfur, chlorine and other elements. White metal with a blue tint.
- Density 10.5 g/cm3
- Melting temperature 960.5 0С
- Hardening shrinkage – 4.4%
- Brinell hardness 26 kgf/mm2
It can be easily processed under pressure due to its high plasticity. It dissolves in hot sulfuric and nitric acids, reacts with hydrogen sulfide and forms silver sulfuric anhydride, silver also oxidizes, has high electrical and thermal conductivity and is part of many alloys (gold, palladium and solders). It has an oligodynamic effect, is used for water disinfection, and is weakened in the presence of salivary proteins.
Palladium (Pd).
Silver-white metal from the group of platinum metals. Has great chemical resistance. In aggressive environments, a passivating film is formed on the surface of palladium, which protects the metal from decomposition. Palladium combines with oxygen only when heated to T 700-900 0C. Palladium is harder than platinum, but is less susceptible to pressure. It has high malleability and rolls well.
- Density – 11.9 – 12.3 g/cm3
- Melting temperature – 1555 0С
- Brinell hardness 49 kgf/mm2
Non-poisonous. Weak oligodynamic effect. Allergen. When working with its compounds, there may be platinosis (cough, sneezing, runny nose, conjunctivitis, urticaria). Palladium is a component of alloys for metal-ceramic dentures and is used for crown moldings in porcelain teeth.
Alloys based on silver and palladium.
PD-250 contains 24.5% palladium and 72.1% gold. Available in the form of disks with a thickness of 0.3 mm and a diameter of 20, 23, 25 mm and a strip thickness of 0.3 mm, used for stamped parts
PD-190 18.5% palladium and 78% ash. In the form of disks Thick 1 mm L 8 and 12 mm. Can be produced in the form of tapes with thicknesses of 0.5, 1 and 2 mm
Used for intermediate parts of bridges and inlays. When melting a PD alloy, especially if it is carried out slowly, the alloy is intensively oxidized and zinc and cadmium evaporate. Therefore, when making a new melt, it is recommended to add at least 50% of the new PD alloy.
Soldering - gold solder, you can use 3lSrKdM - 750 - 30.
Super-T3 gold alloy can be used for stamped and cast prosthetic crowns and bridges, and is intended for veneering plastics and ceramics.
- Melting temperature 880-950 0С
- Hardness N/mm2 (in the cast state 1300-1450, After heat treatment - 2000-2299)
- Yield strength N/mm2 230-250
- Elongation % 20-25
- Density g/cm3 15.2-15.5
- Thermal expansion coefficient (20-600С)*106/0С – 19.7 – 20.3
PLAGODENT (formerly “Super KM”)
Gold-platen alloy. 85% ash, platinum and palladium total 13, copper 1 and tin 1
Used for integrally cast structures (crowns) for applying a ceramic layer
- Melting temperature – 1115 0С
- Vickers hardness – 165 units
- Density – 18.1 g/cm3
produces Palladent TM - a paladium alloy for metal ceramics. Camadent Km for electrochemical coating of dentures. Cadmium-free gold solder – Bekadent.
VITIRIY
. For the manufacture of metal-ceramic and solid-cast denture structures and is produced in the form of strips with a thickness of 2.0 0.1; 2.5 0.1; 3.0 0.1; mm and length 10.0 0.1 mm
Gold 86.7 – 87.7 platinum
Palladium alloy “Superpal” 70% noble metals, for cast crowns and bridge prostheses lined with plastic and ceramics. After polishing, it has a silver-gray metallic color and can be used without veneer.
Base alloys. Titanium and its alloys, alloys based on iron, cobalt, chromium, nickel.
Stainless steels. The steel is resistant to corrosion in the atmosphere, river and sea water.
Chemical properties:
- Formation of a surface protective film.
- Homogeneity of internal structure.
- Lack of phase transformations, which may be cause of microcrack formation.
- Manufacturability.
The most popular stainless steels are 1Х18Н9Т, 20Х18Н9Т, 5Х18Н102G and 36Х18Н25С2. An increase in carbon content to 0.36% leads to an increase in the carbide phase in the pearlite carbide structure; an increase in nickel content to 0.25% increases the proportion of austenitic structure.
Iron (Fe).
Iron in nature occurs more often in the form of oxides and sulfur compounds. Me lilac-silver color
- Density 7.86 g/cm3
- Melting temperature 1535 0С
- Boiling point 2450 0С
- Hardening shrinkage up to 3%
- Brinell hardness 60-70 kgf/mm2
Very flexible and soft material. Chemically very active, in a humid environment it quickly corrodes and becomes covered with a thick layer of oxide. Acids and salts are strong. Depending on the temperature, there can be 4 allotropic modifications.
- Austenite is a solid solution of carbon in iron, characterized by the plasticity of the alloy at a hardness of 200. It is formed at temperatures above 721 0C.
- Ferite is a solid solution of carbon, very soft, plastic, hardness 80 kgf/mm2 according to Brinell. Alpha modification of iron.
- Cementite – Fe3C is very hard and brittle.
- Perlite is a mixture of cementite and ferrite crystals.
Chromium (Cr).
Chromium is contained in steels up to 17-19%, a white metal with a bluish tint, has high corrosion resistance, is brittle and forms carbides with carbon; adding chromium to a steel alloy gives greater resistance and high corrosion properties. Chromium oxide for preparing polishing pastes. When found in steels, it impairs soldering
- Density 7.2 g/cm3
- Melting temperature 1900 0С
- Solidification shrinkage 1.8%
Nickel (Ni).
Occurs in the form of compounds.
The most common are garnerite and arsenic-nickel luster.
Nickel is a shiny metal, can be rolled and drawn well, is resistant to oxidation in air and water, is weakly affected by acids, and is well resistant to alkalis. Has good viscosity and malleability.
- Density – 8.9 g/cm3
- Melting temperature – 14550С
- Boiling point – 29000С
- Hardening shrinkage is very low
- Brinell hardness – 70 kgf/mm2
Additions of nickel to alloys to improve mechanical properties, increase toughness, reduce shrinkage, and impart chemical resistance to the alloy.
Titanium (Ti).
Silver-white metal.
- Density – 4.5 g/cm3
- Melting temperature – 16680С
- Boiling point – 32770С
- Brinell hardness – 100 kgf/mm2
Good corrosion resistance in atmospheric air and water. Forms a protective passive film on the surface. Resistant to acids.
Used for the manufacture of basic dentures.
Titanium alloys are absolutely biologically indifferent; there is no release of nickel and chromium. Technologically accurate material. Easier to get used to the prosthesis. The minimum thickness does not affect the formation of sounds (0.3-0.7 mm).
Also used for braces.
Titanium alloys
Tritan1 – used for all types of work with solid cast structures, bridge frames, bases and arched prostheses.
Remotitan – increased strength and elasticity for clasp and large bridge prostheses.
VT10,VT00
Chemical purity of the alloy is at least 99.5% Ti
Features of modeling titanium structures.
At T 882.5, Ti transforms into a different crystalline structure, and the volume increases to 17%; upon contact with O2, a thin passive layer is formed, which protects against destruction. The anatomical shape is modeled in a reduced form.
For favorable heat exchange between ceramics and Ti, there are cooling ribs or garlands (bridges).
The thickness of the caps is at least 0.4 – 0.5 mm (for casting)
The frames of arched prostheses should be somewhat thicker than when casting from CHS.
Features of processing titanium structures:
- Special cutters with a cross-shaped notch are used.
- If processed incorrectly, there may be: chips, oxidation, overheating of the metal and cracks; the cutter must be driven only in one direction and never go back.
- During processing, the rotation speed of the cutter should not exceed 15 thousand revolutions per minute (or overheating, oxidation).
- Low pressure on the product
- Avoid sharp corners and metal overlaps
- Clean cutters periodically with a steam jet and a fiberglass brush.
Application of KHS alloys.
Arch prosthesis frame and fixed bridge frame, combined prostheses, production of splinting structures, production of solid cast fixed bridges and veneered prostheses.
Modern dental casting alloys.
Base metal alloys:
Materials for heavily loaded denture structures: removable clasp dentures. Splinting devices, bridges, clasps. For products that require increased hardness and elasticity.
Cobalt – chromium – molybdenum is used.
High corrosion resistance and indifference. The yield strength is not less than 500.
Vitalium, Remanium, Virocast, Supercast, Vironit, specially for laser welding Vironium plus, Vironit.
Cobalt-chrome steels
Doynikov 1953.
Base - cobalt has high mechanical properties, chromium to impart hardness and anti-corrosion, molybdenum imparts intercrystalline lattice and increases strength, nickel increases viscosity, molybdenum in small quantities improves casting quality and fluidity, reduces melting point, iron admixture is no more than 0.5%, because shrinkage and physical and chemical properties deteriorate.
Composition of the KHS.
- 26.0% -Chrome
- 6.0% – Nickel
- 0.5% – Manganese
- 0.5% – Mrlibden
- 67.0% – Cobalt
Wironit extrahart from Bego
Auxiliary Me
Copper (Cu).
Copper has a reddish color, ductility, good casting properties, oxidizes in a humid environment and at elevated temperatures, dissolves in nitric, sulfuric acids and alkalis, increases viscosity and mechanical strength, and is used in solders.
- Density – 8.8 g/cm3
- Melting temperature – 19830С
- Boiling point – 23100С
- Shrinkage – 1.7%
- Brinell hardness – 40 kgf/mm2
Zinc (Zn).
The metal is bluish-white in color and is resistant to corrosion because a protective oxide film is formed. It dissolves in hydrochloric and sulfuric acids, has good electrical and thermal conductivity, to increase fluid fluidity.
- Density – 7.2 g/cm3
- Melting temperature – 419.50С
- Boiling point – 9180С
- Brinell hardness – 32 kgf/mm2
- Shrinkage – 0.37%
Cadmium (Cd).
Plastic, soft, easy to forge and roll, dissolves well in acids, and forms a film in a humid environment. For solders and low-melting alloys.
Molybdenum (Mo).
Light gray, refractory, resistant to corrosion, dissolves in aqua regia and nitric acid to produce ferromolybdenum, which is added as an additive in the production of alloy steel.
Aluminum (Al).
Silver-white with a blue tint, the lightest.
- Specific gravity – 2.7%
- Melting temperature 6580C
- Boiling point – 18000С
- Shrinkage – 2.3%
- Brinell hardness – 20 kgf/mm2
Easily stamped, drawn into wire, less viscous than silver, oxide film, not affected by acids, soluble in hydrochloric acid, alkalis and organic acids in the presence of salts. The wire is used for splinting, for jaw fractures, and for orthodontic appliances.
Lead (Pb).
Bluish-gray, shiny, soft, low bending strength, oxidizes in a humid environment, added to easily fusible metal alloys for stamping.
Tin.
Shiny, silvery-white metal.
Good malleability, can be rolled into thin sheets, dissolves in dilute acids, and forms easily fusible metal alloys.
Antimony.
Silver-white with a blue tint, fragile, does not oxidize in air.
Bismuth.
Silvery-white, shiny with a reddish tint, very brittle, resistant to acids.
Nickel silver.
(Melchior).
Low shrinkage, good mechanical properties, for adjusting and repanning devices.
Bronze.
An alloy of copper with precious metals or a copper-aluminum alloy. Golden yellow color. It is used in the form of a wire in orthodontics and maxillofacial orthopedics.
Brass.
Alloy of copper and zinc 1:1. golden color, for museum exhibits.
Corrosion of metals.
Metal corrosion is the process of destruction of metal due to chemical or electrochemical interactions with the external environment.
Reduces the strength and ductility of the metal, spoils its surface, deteriorates the electrical and optical properties, and causes irreversible loss of the metal.
Types of Corrosion:
- Uniform or continuous - a less dangerous type, because with sufficient thickness of the metal, the strength changes little, the surface has a rough or pitted appearance.
- Local – destruction of individual areas of metal in the form of spots and pinpoint lesions of varying depths. In the presence of internal stresses, in case of surface heterogeneity.
- Intercrystalline - destruction along the boundaries of crystal grains, corrosion products accumulate between the crystals, the appearance is not affected, and strength decreases.
Alloys in orthopedic dentistry
Metal occupies a central place among materials in dentistry. Most fixed dentures and removable denture frames are cast (or stamped) from dental alloys. Alloys in dentistry are used as auxiliary materials for soldering and stamping. Dental instruments are made from them.
- Classification of metals and alloys in dentistry
- Structural metal alloys in orthopedic dentistry
- Noble metal alloys in dentistry
- Base alloys in orthopedic dentistry
- Auxiliary metal alloys in dentistry
Primary requirements
The important properties that metals and alloys in dentistry must have are:
- high biological compatibility;
- resistance to corrosion and tarnishing;
- maintaining constant volume and shape;
- safety, no toxicity;
- inertness in the oral cavity;
- mechanical properties (strength, hardness, rigidity, wear resistance, ductility);
- physical properties (density, malleability, thermal conductivity, melting point);
- aesthetic indicators;
- hygienic properties (ease of cleaning with standard dental care products; in the case of using metal dentures, patients should not feel a metallic taste);
- technological characteristics (ease of preparation and processing, segregation).
This is interesting: Dentures with suction cups - types, advantages and disadvantages, before and after photos Alloys
that are intended for ceramic coating must meet the following criteria:
- connects well with porcelain;
- the temperature when processing the alloy should be higher than the temperature when processing porcelain;
- the thermal expansion value of the alloy should be equal to the CTE of porcelain.
An important aspect is shrinkage, which occurs during the transformation of the liquid state of the metal into a solid state.
If the shrinkage is too high, it makes the fabrication of dental structures difficult. Ease of metal casting is important to every dental technician. The lower the temperature when melting and pouring, the easier it is to work with them.
The characteristics of metals must necessarily meet the requirements associated with their purpose and processing methods.
Metals and alloys in dentistry Classification
All metals and alloys are divided into ferrous and non-ferrous.
Ferrous metals are iron and alloys based on it. Steel and cast iron. Cast iron contains more than 2.14% carbon. Not used in dentistry.
The surface of cast iron is matte and non-shiny. It is difficult to polish.
Steel in dentistry
an iron-based alloy containing less than 2.14% carbon. In addition to iron and carbon, steel also contains other metals. They give the alloy new properties (alloy steel), including making it stainless.
Steel copings for stamping crowns
Alloy steel is an alloy of iron and carbon, with the addition of any other metals. They change the properties of the alloy (melting point, hardness, ductility, malleability, etc.).
Alloy steel
Stainless steel is corrosion resistant steel. Chromium (21%), as well as other metals, are most often used as an anti-corrosion agent.
Non-ferrous metals are, respectively, all other metals.
Metals in orthopedic dentistry are divided into noble and non-noble.
Noble metals (or precious metals) are metals that are resistant to corrosion and chemically inert. The main noble metals are gold, silver, and platinum group metals (platinum, palladium, iridium, osmium, etc.).
Base metals are metals that corrode easily and are not found in nature in their pure form. They are always mined from ores.
Purpose
Several decades ago, dentists made medical structures exclusively from noble metals in their pure form.
Gradually, a trend emerged to use them as various alloys. Closer to the 20th century, orthopedic dental appliances made of base metals , in particular alloys of steel and chromium, were common.
Base metals are very hard, which complicates their processing, but thanks to this feature, prostheses can be made of any length.
Pure gold was no longer used due to its insufficient hardness. Today, gold-palladium, gold-platinum, silver-palladium alloys are in demand because they are more durable, resistant to corrosion and have many other positive characteristics.
Base metal alloys are also popular.
They are excellent for ceramic coatings because the oxide film that appears on the surface promotes a strong bond.
Noble metals in dentistry and alloys
Noble metals in dentistry are expensive. But despite this, they continue to be used due to their excellent biocompatibility. They are not subject to corrosion, do not react with saliva, and do not cause allergies or intoxication.
A gold alloy may often be the only option for patients with polyetiological contact allergies.
Noble alloys are durable. Their only drawback (besides the price) is their softness and susceptibility to abrasion.
Gold alloys in dentistry.
COMPOSITION: 90% gold, 4% silver, 6% copper.
PROPERTIES: melting point 1063°C.
The alloy is characterized by ductility and can be easily machined under pressure (stamping, rolling, forging).
Due to its low hardness, the alloy wears off easily. Therefore, when making stamped crowns, solder is poured from the inside, onto the chewing surface or cutting edge.
Produced: in the form of disks with a diameter of 18, 20, 23, 25 mm and blocks of 5 g.
Application: for stamped crowns and bridges made of
alloy of precious metals in orthopedic dentistry
Consists of Gold – 75%, Silver and copper 8% each, and Platinum – 9%
Platinum gives this alloy elasticity and reduces shrinkage during casting.
Used for the manufacture of cast gold parts of clasp dentures, clasps and inlays
- Dental gold alloy 750 standard (ZlSrKdM)
Cadmium is added to the composition - 5-12%.
Due to cadmium, the melting point of the alloy is reduced to 800 C. (The average melting point of gold alloys is 950-1050 C.) Which allows this alloy to be used as solder.
Silver-palladium alloy in dentistry
Silver-palladium alloys are distinguished by a higher melting point = 1100-1200 C. Their physical and mechanical properties are similar to gold alloys. But corrosion resistance is lower. (Silver darkens when in contact with sulfur compounds) Alloys are ductile and malleable. Soldered with gold solder (ZlSrKdM).
COMPOSITION: 75.1% silver, 24.5% palladium, some alloying metals (zinc, copper, gold).
Used for stamped crowns. They are produced respectively in the form of disks of various diameters (18, 20, 23, 25 mm) and 0.3 mm thick.
Composition: 78% silver, 18.5% palladium, other metals.
Used as an alloy for casting in dentistry.
The amount of palladium was reduced to 14.5%, silver was increased.
Application
Crowns, fillings, bridges, dentures, dental instruments, and more are made from all sorts of combinations of alloys and metals.
Materials for creating dental crowns that are especially popular are a combination of metal and ceramics.
A metal base made from alloys of noble metals such as gold, silver, platinum or palladium has a number of advantages compared to a base made from inexpensive materials:
- Gold-based alloys are durable, can withstand heavy chewing loads, have an antibacterial effect, and are unable to cause an allergic reaction, darkening or inflammation of the gums.
- Silver-palladium alloys are more ductile and affordable, but their quality is significantly lower than gold-containing alloys. Temporary crowns are most often made of stainless steel and aluminum.
- Titanium is widely used as a material for creating crowns, solid bridges, and prostheses. Its mechanical characteristics are not inferior to some noble alloys used for dental purposes.
A persistent oxide film is formed on the titanium coating, thanks to which it is corrosion-resistant and biologically compatible with the tissues of a living organism.In addition, it is lightweight, so patients feel comfortable while wearing removable orthodontic appliances with a titanium base.
- An alloy of silver and mercury is used as a filling material. It is quite hard and durable, but raises some concerns due to the mercury content and a possible negative effect on the filling.
Only a highly qualified dentist should place a filling made from such an alloy, since the placement technology presents certain difficulties. - Metals such as nickel, titanium, silver, chromium-nickel corrosion-resistant steels are ideal for the manufacture of implants.
Implants are subject to rejection by biological tissue, as well as deformation, heavy chewing and mechanical loads.Therefore, the materials for their manufacture must have good biological compatibility, strength and corrosion resistance.
- High-hardness tungsten steel is used to make dental cutting instruments. Cheaper steel is used for probing instruments, and dispersion-hardening stainless steel is used for microtools.
Dental stainless steel alloy - dental steel
Steel is the most common alloy in the world. Its properties are well known. And due to alloying agents, it can be given any desired properties.
Dental steel is very cheap.
Among the disadvantages: steel is heavy (density about 8 g/cm3) and chemically active. May cause allergies, galvanosis.
Stainless steel in orthopedic dentistry - grades:
- STEEL GRADE 1 X 18 H 9T (EYA-1)
Dental alloy for crowns COMPOSITION:
1.1% carbon; 9% nickel;18% chromium; 2% manganese, 0.35% titanium, 1.0% silicon, the rest is iron.
Used for fixed prostheses: individual crowns, cast teeth, facets.
- STEEL GRADE 20Х18Н9Т
COMPOSITION: 0.20% carbon, 9% nickel, 18% chromium, 2.0% manganese, 1.0% titanium, 1.0% silicon, the rest is iron.
The following are produced from this type of steel in the factory:
- standard sleeves used for the production of stamped crowns;
- elastic metal matrices for filling, as well as separation strips
STEEL for dentistry GRADE 25Х18Н102С
COMPOSITION: 0.25% carbon, 10.0% nickel, 18.0% chromium, 2.0% manganese, 1.8% silicon, the rest is iron.
APPLICATION: manufactured in the factory:
- teeth (lateral upper and lower) for stamped-soldered bridges;
- frames for metal-plastic bridges, for cladding;
- orthodontic wire with a diameter of 0.6 to 2.0 mm (step 0.2 mm).
Silver solder PSR-37 or Cet solder is used as solder for non-precious alloys
Rina.
Contains silver - 37%, copper - 50%, Manganese - 8-9%, Zinc - 5-6%
Melting point – 725-810 C
Classification
Metals are classified into two main groups - ferrous and non-ferrous. Ferrous materials include iron, iron-carbon alloys, cast iron, and steel.
Non-ferrous - aluminum, copper, zinc, nickel. Modern dentists use more than 400 alloys based on various metals.
They are divided into the following groups:
- Noble.
- Ignoble.
- Solders (precious or non-precious materials used for soldering).
Noble
The cost of noble metals and the alloys they contain is quite high. At the same time, they enjoy deserved popularity because they have an invaluable quality for medical use - biocompatibility.
This is interesting: How to eliminate odor from under a tooth crown: what to do
They are not familiar with the negative effects of corrosion; they do not come into contact with salivary secretions, which are constantly present in the oral cavity and have a detrimental effect on metal structures of a more “primitive” content.
Components made from precious elements do not cause individual intolerance to the material, fraught with outbreaks of allergies, and do not belong to the group of compounds that can cause intoxication as they accumulate in the body.
Due to the described factors, gold or platinum alloys are often the only possible solution for patients prone to polyetiological contact intolerance.
The main advantage of noble metals is their almost lifelong service life. Well, the disadvantages include, perhaps, their too high cost, in comparison with analogue offers made from cheaper components.
The main area of application of noble metals is bridges and stamped crowns. Platinum is an ideal solution for the manufacture of cast-type clasp prosthetic fragments, inlays and clasp retainers.
Properties of noble alloys
The ideal option, according to most dentists, is expensive gold-platinum alloys.
They have impeccable mechanical and physical properties, high biocompatibility and are well combined with ceramics. The most famous noble alloys produced in Russia are “Plagodent” and “Plagodent Plus”. Palladium in combination with gold forms alloys with excellent physical and mechanical properties and high corrosion resistance. They have a decent yield strength and high tensile strength, so long bridges and permanent elements of locking joints are made from them. In appearance they resemble base alloys. Only 10% palladium in the composition gives the alloy a white color, similar to steel.
Working with palladium structures requires certain skills from the dentist due to the high melting point of the alloy and special casting techniques. Soldering and laser welding should be avoided. Gold-palladium alloys are cheaper than gold-platinum alloys: due to different densities, the difference in cost can reach 2-2.5 times.
The percentage of noble metal in the alloy affects corrosion resistance and biocompatibility. The cost of a prosthesis can only be reduced by switching to non-precious alloys. A noble alloy may contain a high or low gold content, or may not contain this element at all, as, for example, in silver-palladium compositions.
What determines the color of noble alloys?
Doctors pay attention to color. It is believed that a very yellow alloy containing a lot of gold improves the color of ceramics: the oxides of such alloys are easily coated with a thin layer of opaque and look aesthetically pleasing. A patient who observes the golden color of the crown frame at intermediate stages has no doubt about the need to purchase such an expensive prosthesis.
In some noble alloys, the proportion of palladium, platinum or silver is increased, so they lose their yellow color, but remain just as strong. Such varieties are suitable for the manufacture of extended bridges, with or without implant support. If you are going to install a single crown, you can get by with an option with a high gold content and a low content of platinum group metals.
Alloys with fewer noble components have a pronounced yellow color and do not have such good properties as white alloys. They contain a high content of indium, which in combination with palladium gives a bright straw color. They do not have sufficient elasticity and corrosion resistance. They are used mainly in China and India on a massive scale to reduce the cost of dentures.
Ignoble
Base metals have a single purpose in their use - reducing the cost of prosthetic structures. They are based on environmentally friendly components that can replace gold or platinum.
Stainless steel, chrome-cobalt combinations of metals, and nickel-chromium compounds are best suited for this.
Such components are the most popular metals in orthodontic practice. In addition, they have excellent alloying characteristics, so that the composition can be given any properties.
This is interesting: Sandwich prosthesis: who will such a prosthesis help and what is hidden behind the “delicious” name
The main advantage of stainless steel is its low cost. Disadvantages can be considered:
- heavy specific gravity - density about 8 grams per cubic cm;
- high chemical activity;
- the ability to provoke allergic reactions;
- tendency towards galvanosis.
Application area:
- fixed type of prosthetics;
- personal crown frames;
- cast dental systems;
- facets;
- sleeves of a standard type, which are a template blank for the manufacture of stamped crown products;
- clasps for fixing dentures and bracket systems;
- filling matrices;
- separation plates;
- soldered bridges;
- orthodontic wire.
Base compounds have good mechanical and chemical properties:
- low density;
- fluidity is within normal limits;
- meltability.
Cobalt chromium alloy in dentistry
(cobalt-chromium alloy, cobalt-chromium alloy)
COMPOUND:
- cobalt 66-67%, alloy base, hard, durable and light metal.
- chromium 26-30%, introduced mainly (as in steel) to increase corrosion resistance.
- nickel 3-5%, increases ductility, malleability, toughness of the alloy, improves the technological properties of the alloy.
- molybdenum 4-5.5%, increases the strength of the alloy.
- manganese 0.5%, which increases strength and casting quality, lowers the melting point, and helps remove toxic sulfur compounds from the alloy.
- carbon 0.2%, reduces the melting point and improves the fluidity of the alloy.
- silicon 0.5%, improves the quality of castings, increases the fluidity of the alloy.
- iron 0.5%, increases fluidity, improves casting quality.
PROPERTIES of KHS dental alloy:
It is distinguished by good physical and mechanical properties, low density (and, accordingly, the weight of restorations) and excellent fluidity, allowing the casting of openwork products of high strength.
Melting point is 1458 C
The alloy is resistant to abrasion and retains a mirror-like shine for a long time.
Cobalt-chrome alloy in dentistry
Used for cast crowns, bridges, solid clasp dentures, frames of metal-ceramic dentures, removable dentures with cast bases, splinting devices, cast clasps.
Metal-ceramics metal composition in dentistry
metal alloy
metal ceramics in dentistry.
Metals
Metals are a group of elements that differ from other groups of substances.
They have variables such as:
— high electrical conductivity; - malleability; — high thermal conductivity; — surface gloss with appropriate processing; — complete opacity; - plasticity.
These aspects are characteristic of both ferrous and non-ferrous metals used in dentistry. Black ones have a dark gray color, their hardness, melting point and density are high.
Colored ones are light (red, yellow, white), more plastic than black ones, less hard, melting points are usually lower.
People of color belong to one of the following groups:
- lungs; - heavy.
The first class includes lithium, barium, beryllium, potassium, sodium, magnesium, calcium, and aluminum. They are characterized by low density in the range of 0.53-3.5. The heavy group includes zinc, tin, copper, nickel, and lead. The density of these metals ranges from 7.14 to 11.34. Titanium is included in the light group, its density is higher - 4.5.
Separately from the colored ones, rare earth and noble ones are distinguished.
These metals are divided into three classes according to their crystal lattice structure:
— the most common is cubic body-centered (vanadium, chromium, molybdenum); — face-centered cubic (lead, copper, nickel); — hexagonal close-packed (zinc and titanium).
Alloys
To obtain a combination of necessary characteristics that one metal cannot provide, an alloy is created from several. There are not only metal alloys, but also non-metallic ones. The former necessarily include a metal or several, or a non-metal and a metal, the latter consist of substances such as glass ceramics, glass and porcelain.
Depending on the number of components, alloys are divided into the following groups:
- binary, if the composition includes 2 elements; - triple - if 3.
And so on according to a similar principle.
The interaction of atoms in an alloy can take place according to several scenarios. Some components, when fused, retain the appearance and characteristics of the grain structure - these are mechanical mixtures. If the particles dissolve in each other, then these are solid solutions. The last group includes most dental alloys, including gold.
Another method of classification is separation by melting point:
- low-melting - up to 300 degrees; - refractory noble - up to 1100; - refractory base - more than 1200.
Nickel-chromium alloys in dentistry
Alloys in which the main element is Ni. The elements of this alloy, in addition to nickel, are Cr (at least 20%), Co and molybdenum (Mo) (4%).
The properties of the nickel alloy are close to the cobalt alloy.
Used: for casting fixed dentures and removable denture frames.
Today, the use of nickel alloys is limited due to their high allergenicity.
Titanium alloys in orthopedic dentistry
In dentistry, both pure titanium (99.5%) and its alloys are used.
The use of orthodontic wire in modern medicine
Until the 50s of the last century, orthodontists used only precious metals for their purposes. Other materials did not meet safety parameters for humans or did not withstand the internal oral environment. The emergence of orthodontic wire with a wide range of mechanical properties and at the same time a high degree of resistance to corrosive influences in the oral cavity has made it possible to solve many problems and reduce the cost of manufacturing springs and rings in removable devices, as well as braces and arches.
Basic properties of orthodontic wire
- Corrosion resistance;
- Strength and elasticity. Kink resistance;
- High stability of mechanical properties;
- Extreme accuracy of parameters.
These properties allow for great variation through cold working and annealing. Stainless steel wire is softened by annealing and hardened by cold working. The resulting material has high elasticity and deformability.
Dental orthodontic stainless steel wire
Dental wire with a diameter of 0.6 or 0.8 mm is used as ligature wire. Wider diameters (1 mm or 1.2 mm) are used for the manufacture of removable dentures and maxillofacial devices.
In the manufacture of orthodontic prostheses or devices, there is often a need to connect individual elements or structures by welding or soldering. It is important to understand that prolonged exposure to high temperatures can change the properties of stainless steel, therefore the technologies that will be used to connect elements must eliminate prolonged exposure to high temperatures on metal parts of the structure. Typically, stainless steel elements are joined using hard solder.
Auxiliary alloys used in orthopedic dentistry
Bronze is an alloy of copper and tin. Aluminum bronze (aluminum instead of tin) is used in dentistry. It is used to make ligatures for splinting jaw fractures.
Brass is an alloy of copper and zinc; pins for collapsible models are made from it.
Magnalium is an alloy of aluminum and magnesium; aircraft parts are made from it (the alloy is very light and durable). In dentistry, articulators and some cuvettes are made from it.
Amalgams are an alloy of metal and mercury. Used for filling.
The topic is too broad; amalgam in dentistry will be discussed in a separate article.
Low-melting alloys in orthopedic dentistry
Low-melting alloys (Mellota, Wood, Rose) - contain Bismuth, Tin, Lead
– their melting point is about 70 C.
They are used for dies when stamping crowns, counter dies, and making collapsible models.
Low-melting metals in dentistry
Wood's alloy.
Melting point 68 C.
Composition: Bismuth – 50%, Lead – 25%, Tin – 12.5%, Cadmium – 12.5%.
Toxic because it contains cadmium.
Mellot alloy.
Melting point 63 C
Composition: Bismuth – 50%, Lead – 20%, Tin – 30%.
Rose alloy for dentistry.
Melting point 94 C.
Composition: Bismuth – 50%, Lead and Tin 25% each.
Definition of structure
Using microscopic and macroscopic analyses, you can find out the structure of metals. Macroanalysis helps determine:
- micro- and macroscopic metal defects;
- chemical composition (heterogeneity of various alloy elements);
- structure (heterogeneity of alloys resulting from heat treatment).
Thanks to the microscopic method of examination, it is possible to establish:
- the shape and dimensions of any structural elements of the alloy;
- microcracks that violate the integrity of the material;
- structures typical for certain processing methods;
- additives of non-metallic origin;
- a change in structure that occurs under thermal influence.
Steel for dental instruments
Tool steel – contains carbon from 0.7% or more.
It is characterized by high strength and hardness (after special temperature treatment).
The addition of tungsten, molybdenum, vanadium and chromium to steel makes the steel capable of cutting well at high speeds. This steel is used for burs and cutters.
Tungsten carbide is not an alloy. A chemical compound of tungsten with carbon (chemical formula WC). Comparable in hardness to diamond. Used for the production of armor-piercing tank shells. And also for carbide dental burs.
Zirconium metal in dentistry
Zirconium dioxide is also not an alloy. Chemical compound of zirconium metal with oxygen. Its chemical nature is similar to ceramics, but it is harder and more durable. In dentistry they are used for the manufacture of milled dentures.