Margarita Morozova “Popular Mechanics” No. 3, 2018
The shock and awe of visiting the dentist is familiar to us since childhood. And many adults feel overwhelmed by the jingling of instruments, and sometimes by the mere sight of a dental clinic. As a result, despite all the successes of modern medicine, there is practically no hope for a future without caries. But it is this, together with periodontitis, that is the main cause of tooth loss in people of all ages. The problem stimulates the search for new treatment methods, including in the field of bioengineering. Methods that will allow you to forget about fillings and crowns and simply grow new healthy teeth instead of damaged ones.
Tissue engineering in dentistry has been used since the era of the pharaohs: the oldest known dental implants were found by archaeologists in Egypt. Among them are teeth that were reimplanted into the woman in place of the lost ones and were partially integrated with living tissue. An artificial tooth was discovered in a man's jaw, expertly carved from a mollusk shell 5,500 years ago. But despite the impressive period, complete treatment for a patient with adentia, that is, complete or partial loss of teeth, still does not exist.
Background
around 330 BC Aristotle describes the regeneration of the tip of a lizard's tail. 1950s Two types of stem cells are found in the bone marrow: hematopoietic precursors of blood cells and mesenchymal precursors of bone and cartilage tissue, including teeth. 1981 For the first time, mouse embryonic stem cells have been isolated and grown in vitro. 1985 Stem cells have been found in human dental pulp. 1998 Successful isolation and laboratory cultivation of human embryonic stem cells. 2000 It has been shown that mesenchymal stem cells in the pulp are capable of regenerating dentin-like tissue complexes. 2003 It is possible to isolate a population of stem cells from the still living remains of a decayed tooth. 2004 Finding stem cells in the periodontal ligament, which holds the tooth in place. 2006 Mature differentiated cells from mice have been successfully “reprogrammed” into stem (induced pluripotent) cells. 2007 Induced pluripotent stem cells are derived from human fibroblasts. 2010 Dentin-like complexes were grown from pulp stem cells on an artificial scaffold.
Competition "bio/mol/text"-2017
This work was published in the “Free Topic” category of the “bio/mol/text” competition 2017.
The general sponsor of the competition is: the largest supplier of equipment, reagents and consumables for biological research and production.
The sponsor of the audience award and partner of the “Biomedicine Today and Tomorrow” nomination was.
"Book" sponsor of the competition - "Alpina Non-Fiction"
...They say evil has no face. Indeed, no feelings were reflected on his face. There was not a glimmer of sympathy on him, but the pain was simply unbearable. Can't he see the horror in my eyes and the panic on my face? He calmly, one might say, carried out his dirty work professionally, and at the end he politely said: “Rinse your mouth, please...”
This is how Dan Andrews describes a visit to the dentist in his short story “Wretched.” Indeed, since childhood, we have been in incredible awe of such specialists as dentists. Parents do everything they can to force their children to at least go to the doctor’s office, trying not to think about what awaits them next. And sometimes an adult’s soul sinks at the sight of numerous tools. Sometimes just the sight of a dental clinic is enough to do this.
As a result, the state of the oral cavity and dental hard tissues around the world does not inspire hope for a future without caries. Despite advances in dental treatment, tooth loss remains one of the most significant problems. Thus, according to WHO, the main causes of tooth loss are caries and periodontitis. Complete tooth loss is particularly common among older people. Globally, approximately 30% of people aged 65–74 years are missing teeth due to inflammatory periodontal diseases and pathology of dental hard tissues [1].
Therefore, it is not surprising that the state of the oral cavity of the population not only in Russia, but also in the world represents a serious problem, offering opportunities both for study and, more importantly, for the search for new treatment methods. One of them was tissue engineering , an interdisciplinary branch whose goal is to create biological substitutes that restore and maintain the functions of a tissue or organ. The fairly high efficiency of tissue engineering methods and their potential have attracted the attention of many scientists. This also contributes to their unfading popularity in various fields of medicine to this day.
Will a new tooth grow if you really want it?
Supporters of spiritual practices are confident that it is possible to provoke the appearance of new teeth if you call on them with the power of thought. To do this, you need to transfer to the body a detailed thought form of the object that you would like to grow naturally.
As a guide and incentive for the experiment, you can use the book by Mikhail Stolbov, in which he talks about how he managed to grow 17 new teeth.
In the book, the author tells his story, which began while serving in the army. A young soldier had almost all his teeth knocked out with a stool in a fight.
In the years following the army, Mikhail Stolbov constantly changed his dentures. He came to terms with his defect. But, as fate would have it, the man was literally locked up for a year in the Siberian taiga. In forced confinement, he developed a disease that made it impossible to wear prosthetics.
After this, Mikhail began to think about alternative ways to solve his problem. And he found a way out. Stolbov no longer wears dentures. He grew 17 new snow-white teeth, contrary to current medical dogma.
The man does not know what was the impetus for the wonderful event.
Tooth for tooth
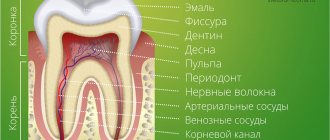
People made their first attempts at dental treatment a long time ago. During excavations in Egypt, archaeologists discovered an artificial tooth carved from a mollusk shell in the jaw of a man who lived five and a half thousand years ago (Fig. 1) [2].
Figure 1. View of the vestibular surface of a tooth cut from a shell. The interval of marks along the edges is 1 mm.
[2]
In addition to the “seafood tooth,” they found reimplanted teeth in the jaw of a young woman, and all of them were out of place: instead of the upper central incisor, the alveolus contained a fang. These teeth had all the signs of integration, that is, fusion with living tissue [3]. Thus, it turns out that already at this time the first steps were taken in dentistry, but, more surprisingly, also in the field of tissue engineering.
But, you ask, how can dentistry and tissue engineering be related, apart from the fact that several thousand years ago an Egyptian valued his smile so much that he replaced a lost tooth with someone else's? They very well may, because at the moment there is no panacea for treating a patient who has been diagnosed with partial or complete adentia , that is, lack of teeth. In addition, the loss of even one tooth leads to changes not only in aesthetic parameters, but, more importantly, to disruption of primary food processing and deterioration of speech. We should also not forget that when teeth are lost - whether as a result of injury or caries and its complications - the condition of the dental system as a whole changes, which worsens the prognosis and complicates further treatment.
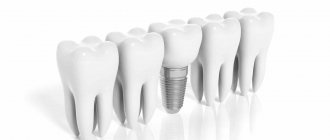
In order to compensate for the functions of a lost tooth, orthopedic structures and implants are now used (Fig. 2). Still, these are “artificial” substitutes: they lack blood vessels, nerve endings, and receptors. Also one of the most important aspects is the absence of the periodontal ligament in the implant, which until recently was considered the gold standard of treatment for missing teeth.
Figure 2. Structure of the tooth and implant. Natural tooth - tooth. Artificial crown - artificial crown. Gingiva - gums. Implant - implant. Osteointegration - osseointegration. Periodontal ligament - periodontal ligament.
website www.neoclinique.ro
The periodontium is a highly specialized fibrous connective tissue composed of cells and extracellular matrix. It is located between the cement that covers the root of the tooth and the bone tissue that forms the wall of the socket. In humans, the periodontal ligament helps strengthen the tooth in the alveolus, provides mechanical resistance to the effects of chewing forces on the tooth, distributing the applied pressure: the force of all masticatory muscles is no less than 390 kg [4].
What's wrong with the implant?
Firstly, as already described above, there is the absence of the periodontal ligament. The implant is retained due to osseointegration, that is, through an anatomical connection with bone tissue. Unlike a tooth, which has little physiological mobility, an implant is immobile. If a semblance of connective tissue appears around the implant, then this means only one thing - peri-implantitis , that is, an inflammatory process in the bone tissue surrounding the implant. In most cases of this scenario, the implant must be removed [5].
Secondly, the implant cannot be connected into a common structure with the patient’s remaining teeth due to the lack of ligamentous apparatus and the inability to adequately distribute pressure. The principle works here: whoever is stronger is in the dentition. Either the implant will not allow the tooth to move, which will lead to atrophy of periodontal tissue and tooth loss, or the implant will be lost.
Thirdly, each patient has its own anatomical features, and the volume of bone tissue for placing an implant is not always sufficient.
And fourthly, it is important to remember that for the longevity of the implant it is necessary to maintain ideal oral hygiene, which, to put it mildly, is not possible for everyone. Here we return to the previously mentioned problem of peri-implantitis [5]. It turns out to be a kind of vicious circle.
All these disadvantages lead to the search for alternative treatment methods.
One of them could be tissue engineering. In this article, I will try to summarize the recent progress, prospects and main directions of development of dental bioengineering, that is, briefly talk about what it takes to create a tooth.
Examples of people growing new teeth
There are cases of spontaneous regeneration in middle-aged and older people.
A real medical sensation was recorded in Rostov-on-Don. A young 28-year-old woman, Evgenia Baykova, has grown 33 teeth. This fact was officially certified by local dentists. Evgenia claims that she grew it through willpower.
In the Kazakh city of Kyzyl-Orda, a hundred-year-old grandmother erupted new teeth. This sensational message was broadcast on television. The woman’s rejuvenation process, according to her relatives, began with a rise in temperature. By the way, he also touched his hair. Among the strands that had long since turned gray, she had completely black curls.
There is also other evidence of people suddenly growing new teeth. An example of this phenomenon can be seen in the video.
What is the secret of such amazing behavior of the body? What triggers the natural restoration of organs?
After all, if there is a way to grow new teeth, then why endure the pain of going to the dentist and paying a lot of money for installing implants?
Where do teeth come from, or odontogenesis in vivo?
Naturally, before understanding bioengineering, you need to understand how a tooth initially develops in the human body.
The formation of teeth is a rather complex process, which is accompanied by tissue interaction and controlled by a huge number of signaling molecules (Fig. 3) [6].
Figure 3. Stages of tooth development. During tooth development, the tooth goes through the following stages: placode, bud, cap, bell, root development and eruption. Tooth formation begins in the area of the dental plate, which consists of mesenchymal cells and invaginated epithelium. At the first stage, a tooth germ is formed from the dental plate (placode stage). During the cap stage, the primary enamel node is formed, and at the bell stage, secondary enamel nodes are formed, which form the cusps of future tooth crowns. Here, the epithelial and mesenchymal cells of the tooth embryo differentiate into ameloblasts, odontoblasts and dental follicle cells. Ameloblasts and odontoblasts produce enamel and dentin, respectively. Dental follicle cells differentiate into periodontal tissue cells: periodontal ligament, cementum and alveolar bone.
[7]
The tooth develops from tissues formed by the germ layer ectoderm. By dividing and differentiating, ectoderm cells form the structures necessary for tooth development: the dental epithelium and neural crest, which later transforms into mesenchyme. Tooth formation is initiated and regulated by epithelial-mesenchymal interactions. The earliest sign of tooth development is the formation of the dental lamina, a horseshoe-shaped thickening of the epithelium along the upper and lower jaws. Further stages include placode, bud, cap, bell and root development [6], [7].
The interaction between epithelial and mesenchymal cells plays a major role in tooth development. Why, during the development of the embryo, is it the tooth that is formed, and not another organ, for example, the intestines? The thing is that the cells involved in tooth development have odontogenic competence. The genetic background of odontogenicity, that is, the ability of stem cells to differentiate directly into dental cells, is not fully understood, although more than 200 genes “involved” in tooth development have been identified. Many studies aimed at studying this phenomenon also pay a lot of attention to certain epithelial signaling centers. In total, we currently know about 4 such centers: the dental plate, placode, primary and secondary enamel nodes, the main role of which is the expression of signaling molecules that regulate tooth formation [8], [9].
Gene stimulation will help you get a beautiful smile
Some geneticists believe that by stimulating certain genes, people can grow new teeth and naturally repair damaged hard tissue. After all, it is known that at birth and in childhood, teeth grow with the help of a certain gene, but in adulthood this gene simply stops “working.”
Scientists from Zurich have already identified genes (Msx1 and Osr2), as well as chromosome regions responsible for the formation, development and even the correct position of the crown.
Many scientists are working on gene stimulation
Geneticists, especially from the USA, are actively applying this knowledge in practice. Texans, for example, managed to grow teeth in vitro by controlling the activity of certain genes. And scientists from Oregon have achieved enamel regeneration by influencing the structure of DNA.
Scientists cannot yet answer the question of what the invasion of human genetics will lead to. It is likely that progress in this area will result not only in positive achievements, but also in various mutations and failures.
Non-Hong Kong "Triad"
Now that we know so much about the origin and development of the tooth, we can move directly to the topic of interest to us - tissue engineering.
Tissue engineering is a set of methods and procedures aimed at the regeneration of biological tissues. It includes a triad of main elements (Fig. 4): stem cells, extracellular matrix or scaffold, growth factors and signaling pathways [10].
Figure 4. Tissue engineering triad. The basis of the tissue engineering triad is stem cells, growth factors and extracellular matrix.
[10]
The goal of tissue engineering is to replace lost cells, tissues and organs, or promote their regeneration, or simply restore impaired function.
Today we hear and read a lot about stem cells. This is a hotly debated branch of science. The information that goes out to consumers, as a rule, is not always objective. What exactly are stem cells, and how and which of them can be used in dental tissue engineering?
Let's get acquainted: stem cells are undifferentiated embryonic or adult (postnatal) cells that are capable of going through a huge number of cell divisions while in an undifferentiated state, as well as forming intermediate cell types - precursors that can differentiate into various cells and create full-fledged tissues and organs (Fig. 5) [10], [11].
Figure 5. Classification of stem cells according to their ability to differentiate. Based on the scale of differentiation, stem cells are divided into totipotent, pluripotent, multipotent and unipotent. Totipotent cells are capable of differentiating into any cell type of an adult organism. Pluripotent cells can produce specialized cells of the three germ layers (ectoderm, endoderm and mesoderm), but not the entire organism. Multipotent cells produce a limited range of cell types. Unipotent cells are capable of differentiation into only one type of cell [13].
[11]
The first cell line of embryonic stem cells was isolated back in 1998 [12]. In fact, not so long ago, and from the point of view of the course of history one can say quite recently, but the progress is colossal [10].
Embryonic stem cells are isolated from the blastocyst during embryonic development. They give rise to three germ layers: ecto-, endo- and mesoderm. These cells are totipotent, meaning they can develop into each of the more than 200 cell types in the adult body [10].
There are currently 3 known sources of mammalian embryonic stem cells: cells isolated from the inner cell mass of the blastocyst; teratoma cells and primary germ cells of the embryo [10].
As was previously mentioned, stem cells are not only embryonic, but also postnatal. As for “adult” stem cells, they exist in the body in various tissues, including bone marrow, blood vessels, liver, skin, adipose tissue and dental tissue. They are localized in special niches where their proliferation, migration and life span are regulated. Postnatal stem cells are multipotent, meaning they give rise to only one type of cell.
Dental stem cells are a population of postnatal mesenchymal stem cells (MSCs) that have the ability to self-renew and differentiate [4], [14]. Depending on the location of the MSC depot (Fig. 6) [15], they are divided into:
- pulp stem cells;
- apical papilla stem cells;
- stem cells from extracted baby teeth;
- dental follicle progenitor cells;
- periodontal ligament stem cells;
- MSCs obtained from the alveolar process;
- MSCs of the gums;
- progenitor cells (MSCs aimed at differentiation only into a certain type of cell) of the tooth germ.
Figure 6. Dental stem cells. Schematic representation of sources of dental stem cells. For an explanation of the abbreviations, see the box below.
[15]
Abbreviations
WHO World Health Organization MSCs mesenchymal stem cells ECM extracellular matrix ABMSCs alveolar bone-derived mesenchymal stem cells BMP bone morphogenetic protein DFPCs dental follicle progenitor cells DPSCs dental pulp stem cells FGF fibroblast growth factor GMSCs gingival mesenchymal stem cells iPSCs induced pluripotent stem cells PDGF platelet derived growth factor PDLSCs periodontal ligament stem cells SCAP stem cells from the apical part of the human dental papilla SHEDs stem cells from human exfoliated deciduous teeth TGPCs tooth germ progenitor cells germ progenitor cells)
Let's look at some of them.
Pulp stem cells can be quite easily isolated from the pulp of extracted teeth. They represent a very attractive and promising source of autologous stem cells and can be used both for the regeneration of dentin, pulp and cement, and for the restoration of bone tissue [15]. In addition, they exhibit strong neuroregenerative activity, which is of particular value in the treatment of spinal cord injuries: pulp MSCs, in addition to suppressing the early inflammatory response, inhibit the apoptosis of neurons, astrocytes and oligodendrocytes after injury, which leads to the preservation of the nerve fiber and myelin sheath. They have also been found to promote the regeneration of severed axons. Thus, scientists hypothesize that pulp MSCs could provide significant therapeutic benefits in the treatment of spinal cord injury [16].
Stem cells from extracted primary teeth are a postnatal population of stem cells with high proliferative capacity, high viability, and the potential for multilineage differentiation (e.g., into osteoblasts, neuronal cells, and odontoblasts) [15].
Gum mesenchymal stem cells are ideal for restoring damaged periodontal tissue, muscles and even tendons. But it is not yet entirely clear whether they are capable of forming dentin and pulp cells [15].
Tooth germ progenitor cells are a relatively new population of stem cells that were discovered in the mesenchyme of the third molar germ at the bell stage. They show the same multilevel differentiation as other dental MSCs, including the ability to differentiate into adipocytes, osteoblasts, odontoblasts, chondrocytes and neurons, and can also differentiate into cells with the morphological, phenotypic and functional characteristics of hepatocytes. Hence, it is assumed that this type of stem cells can be used in the future to treat liver diseases [15].
Thus, each type of dental stem cells has its own characteristics and areas of application not only in dentistry, but also in other areas of medicine.
In addition to the MSCs described above, induced pluripotent stem cells (iPSCs) derived from somatic cells are also used in tissue engineering. They were first discussed in 2006, when Japanese scientists Kazutoshi Takahashi and Shinya Yamanaka showed that somatic cells can be reprogrammed into iPSCs by increasing the expression of certain transcription factors (Oct3/4, Sox2 and Klf4) [17], [18]. These cells themselves are immunologically neutral and, just as importantly, do not raise the same ethical controversy as embryonic stem cells. However, viral agents were used to reprogram them, which could lead to the formation of neoplasms [19]. There were attempts to use chemical molecules instead of viruses [20], but, unfortunately, the percentage of successful reprogramming turned out to be small. New methods for obtaining iPSCs are now being developed, since their application looks quite attractive and very promising.
The other side of the issue
But every movement also has an opposite direction. This explains the phenomenon of a rare disease - progeria (rapid aging of people). You've probably noticed that illnesses age people faster than time, and you've seen that the dead age several years in a matter of hours.
The sooner the work begins, the sooner the result will be. Devices for restoring teeth (and healthy organs) can be made in any country: Russia, Japan, USA, Germany, Israel, Canada, China, Italy, Sweden and others where there is appropriate equipment.
There is also a large army of opponents: dentists, prosthetists, pharmacists, “scientists”, corrupt officials, envious people and others who may lose income from the presence of healthy people in the world. The country, institute, entrepreneur who helps and begins to develop this project will receive profits and gratitude from all humanity for years to come.
What does it cost us to build a tooth?
To use stem cells in tissue engineering, the presence of a scaffold and growth factors is required (Fig. 7). An ideal scaffold should support cell attachment, migration, proliferation, and spatial organization.
Figure 7. What does it cost us to build a tooth?
website dentistry.tamhsc.edu
Basically, a scaffold as a suitable matrix for tissue reconstruction should meet the following requirements [21]:
- Ease of use.
- The presence of pores of a certain shape and size for the diffusion of cells, growth factors, nutrients and removal of waste products.
- The ability to biodegrade, which occurs at a certain time without releasing toxins.
- Biocompatibility with body tissues.
- Low immunogenicity.
- Ability to be replaced by regenerating tissue and vascularization.
- Good physical and mechanical properties.
The materials used to form scaffolds are divided into natural and synthetic (Fig. [22]. Bioactive glass, polylactic acid, various composites (multicomponent materials based on a matrix based on metal, polymer or ceramic) - all these are synthetic materials. Despite the fact that these materials make it possible to produce scaffolds of the required shape, their use is very limited due to unsatisfactory biocompatibility and toxicity. Among the biomaterials (natural materials) used to create scaffolds, collagen, chitosan, and hyaluronic acid can be distinguished. They consist of macromolecules that They are also part of the extracellular matrix, therefore they are biocompatible and highly biodegradable, but they are less durable and can cause rejection reactions [21].
[22]. Bioactive glass, polylactic acid, various composites (multicomponent materials based on a matrix based on metal, polymer or ceramic) - all these are synthetic materials. Despite the fact that these materials make it possible to produce scaffolds of the required shape, their use is very limited due to unsatisfactory biocompatibility and toxicity. Among the biomaterials (natural materials) used to create scaffolds, collagen, chitosan, and hyaluronic acid can be distinguished. They consist of macromolecules that They are also part of the extracellular matrix, therefore they are biocompatible and highly biodegradable, but they are less durable and can cause rejection reactions [21].
Figure 8. 3D scaffold of mouse and human teeth. a — Lower central incisor of a mouse. b — Human lower first molar. 3D reconstruction and bioprinting were used. Material: hydroxyapatite and polycaprolactone. Microchannels (d = 200 nm) into which MSCs and growth factors are introduced ( c and d ) are visualized.
[22]
The most suitable scaffold that meets most requirements is either a scaffold derived from extracellular matrix ( ECM scaffold ) or its analogue. Due to their identity with the extracellular matrix, such scaffolds are able to provide the best interaction with cells and growth factors. Dental MSCs, such as pulp and periodontal stem cells, when cultivated in ECM scaffolds, underwent differentiation in the odontogenic direction. After implantation of this scaffold, the pulp was formed [10], [23].
In addition to the scaffold and stem cells, a link is needed that connects them, which would regulate tissue growth. These can be growth factors, certain genes, and interfering RNAs [7].
Growth factors are peptide molecules that transmit signals to control cellular behavior and interact with specific receptors on the surface of cells [24]. They provide interconnection and interaction between cells and the extracellular matrix. Following cell damage, the secretion of growth factors begins, which subsequently trigger the processes of regeneration and angiogenesis. An example of the “work” of growth factors in a tooth is the formation of secondary and tertiary dentin, which occurs when the carious cavity is close to the dental pulp or when teeth are subject to increased abrasion. Key growth factors during tooth development include bone morphogenetic protein (BMP), platelet-derived growth factor (PDGF), and fibroblast growth factor (FGF). They are primarily used in dental tissue engineering [25–27]. Both cells and nanoparticles, as well as the scaffold itself, can be used to deliver growth factors.
The recipe is ready
That's all, in short, what is needed to create teeth. Thus, the recipe for creating a tooth looks something like this:
- Stem cells - assorted
- Scaffold is a natural product
- Growth factors - to taste
Regenerative medicine technologies are progressing incredibly quickly. And now, probably, the most basic provisions for dental tissue engineering have already been developed. They all stem from our knowledge of the cellular and molecular basis of tooth development. We understand that the best result in tooth bioengineering can be achieved only in the presence of two types of cells, and not one: these are both epithelial cells and mesenchymal cells (where would we be without them?) [28]. However, you cannot build a tooth on cells alone. Thus, the role of growth factors and extracellular matrix cannot be excluded here. Fortunately, science does not stand still, and new provisions are being actively developed. Perhaps, in the near future, the treasury of knowledge called “dental tissue engineering” will be replenished with another equally valuable “coin”.
But, despite all the promising potential of tissue engineering in dentistry, there are still problems to be solved related to the conduct of clinical trials, the innervation and blood supply of the bioengineered tooth, its ligamentous apparatus, the timing of its eruption, as well as the choice of a pool of stem cells and technology for working with them , and a number of other equally pressing tasks [10], [29].
As for the most basic thing, namely stem cells: in the experiments performed (it is worth noting that almost all of them were carried out on mice), they mainly used embryonic stem cells. But in the clinic their use is sharply limited, including by law. Therefore, only postnatal stem cells remain (not counting iPSCs, where things are also not calm), and here we face the following snag: unlike mice, humans lack a niche of dental stem cells, which is why our teeth do not have the ability to constantly grow. Those MSCs that are suitable for use cannot be obtained without damaging the tooth, or even more so if the tooth was previously treated endodontically, that is, with pulp removal. Those to which access is open do not have odontogenic competence, for example, gingival MSCs. This is just one of the dilemmas that remain to be resolved (Fig. 9).
Figure 9. The fight for healthy teeth for humanity.
website accuratedentistry.net
Treatment of body diseases is the key to dental integrity
Doctors of medical sciences at the Central Research Institute of Dentistry believe that the destruction of the enamel-dentin layer may be associated with the disease and condition of a specific organ. If you maintain general health and promptly treat internal diseases of the body, your teeth can be successfully preserved.
A number of researchers believe that the incisors on both jaws are interconnected with the organs of the kidneys and bladder. The canines can be affected by the condition of the liver and gallbladder, while the premolars and molars can be affected by the intestines, lungs, spleen, stomach and pancreas. And “eights” have a connection with the heart and small intestine. According to this theory, the state of certain elements of a series can signal problems with internal organs and vice versa.
Forward to the future!
Of course, there is no doubt that dental bioengineering will soon become an integral part of standard protocols for the treatment of dental lesions. It is possible that regenerative dentistry techniques will allow us to create a complete dentogingival complex. It is important to remember that methods developed in accordance with the requirements and objectives of dental bioengineering will be able to spur the development of new approaches to the regeneration of other tissues and organs and thus contribute to progress not only in dentistry, but also in the field of regenerative medicine in general. Well, forward to the future!
Preserving lost teeth – a chance for a new smile
Scientists believe that the maximum benefit can be obtained from a mature tooth if it contains living pulp. According to a number of studies, the nerve bundle contains a large concentration of substances that in the near future will be useful for growing new teeth from stem cells. Therefore, if a tooth falls out or has to be removed, then professors suggest saving it for practical purposes, so to speak, for the future.
As far as milk units go, they are a storehouse of stem cells. Scientists believe that in the future it will be possible not only to successfully regenerate new elements from them, but also to restore existing ones. Also, with their help, it is planned to learn how to have a beneficial effect on other organs and tissues. Californian professors have already proven that stem cells from a number of dairy elements help synthesize pancreatic tissue.
Scientists advise saving dropped units
There are still no banks for storing these valuable “human” materials, but scientists advise not to despair, but to place the fallen units in the freezer. It is best to do this no later than 48 hours after loss or removal. The greatest potential of stem cells is in children who have not yet turned 8-10 years old.
Researchers are confident that stem cells located in the tooth are capable of independently restoring hard tissue, but for this they must be properly activated. To confirm their guesses in practice, experts created an imitation of caries in experimental mice by drilling holes in the enamel-dentin layer and filling them with stimulating drugs. After just six weeks, complete regeneration of hard tissue occurred due to the production of a large amount of dentin.