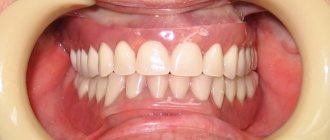
Belak F is a one-component varnish containing fluorine. Its regular use reduces tooth sensitivity.
This drug also helps to increase the resistance of enamel to caries and is used to remineralize enamel.
Features of the composition
Belak F is produced in 2 types:
- white;
- transparent.
White varnish contains:
- solvent;
- film former;
- potassium fluoride.
The transparent version of the drug contains:
- film former of natural origin;
- new generation fluorine compound – aminofluoride;
- antiseptic;
- solvent.
Belak F is produced in a 25 ml dark glass bottle. The kit may also include applicators for application.
Compound
- natural film former;
- aminofluoride (new generation fluorine);
- antiseptic component and solvent;
- fluoride ions.
If you get used to it, you can apply it in four stages: treat the upper teeth on the right completely, then on the left. Accordingly, the lower ones are the same. It will take a lot of time, but you will be confident that you are doing everything right.
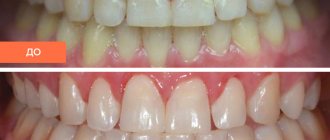
For those who have sensitive teeth, Belak F for teeth is simply a salvation, especially if courses are carried out regularly 2-3 times a year for the purpose of prevention, but it is better to discuss treatment with your dentist. An integral advantage is also that the material consumption is not great, and it is enough for a long time, especially for such a price. One vessel is enough for more than 4 times, which means the treatment will cost a pretty penny, unlike the paid sessions that dental clinics offer us.
Mechanism of action and therapeutic effect
The preventive effect of the varnish is based on the penetration of fluoride ions into the enamel hydroxyapatite crystals. At the same time, their solubility decreases. Fluorine becomes an integral element of the crystal lattice, thereby reducing the rate of demineralization processes and increasing remineralization.
Fluoride exposure lasts longer due to the addition of fluoride ions to calcium ions. The resulting substance fills all the pores and microchannels in the enamel where there is the highest probability of reducing its resistance.
In addition, the influence of microorganisms on the enamel is reduced. When fluorine-containing preparations are applied, they lose their ability to release acids when processing sugars.
The result of the cariesstatic effect of fluorine-based varnishes is a decrease in the intensity of carious processes in 60% of cases.
Increased tooth sensitivity occurs due to the fact that the enamel is worn away in one or more places on the tooth. As a result, the dentinal tubules become open, which causes discomfort.
The effect of fluoride varnish on such teeth is based on their covering with poorly soluble calcium and fluoride compounds. The crystals are deposited in the tubules and ensure their hermetically sealed.
The use of caries-preventive drugs for local prevention of dental caries
G. M. Fleisher , consultant physician, State Healthcare Institution "Regional Dental Clinic - Dental Center", two-time record holder of the Guinness World Book of Records (Lipetsk)
The use of caries prophylactic drugs is today one of the few scientifically substantiated and proven methods of effective fluoride prevention. Expanding our knowledge and understanding of the mechanisms of the protective action of fluorides has largely changed the attitude towards various methods of fluoride prophylaxis.
The use of caries-preventive oral hygiene products currently forms the basis of any caries-prevention program, both at the individual, group, and community levels.
A number of innovative approaches, such as the use of organic fluoride-containing compounds and the slightly acidic pH value of the oral hygiene products used, can significantly enhance their caries-preventive and remineralizing effects.
Fluoride preparations, proposed in 1939 and successfully used to this day, have become widespread in dentistry (Markskors D., 2003). The effect of fluorine preparations is based on the formation of CaF2 and fluorapatite crystals with a crystal lattice more resistant to acids. Fluoride ions and their role in increasing the resistance of enamel and dentin are the result of decades of research and thousands of publications.
The result is that every year fluoride compounds are becoming more and more effective in the treatment and prevention of various diseases and pathological conditions of the teeth (M. Nanaka, K. Matsunaga, Y. Kadoma, 2000). Accordingly, widespread caries prevention strategies aim to incorporate fluoride into the enamel structure, primarily during the pre-eruption stages of tooth formation.
The most common methods of systemic (or endogenous) fluoride prophylaxis, designed to increase the proportion of fluoride-containing apatites in the enamel structure, include the use of fluoride in tablets and fluoridation of drinking water. Clinical studies supporting the above concept are based on three groups of clinical studies:
- caries-preventive effect of systemic administration of fluoride-containing drugs during pregnancy;
- changes in the intensity of dental caries in children living in regions where drinking water is fluoridated;
- “Reducing the solubility of tooth enamel due to the incorporation of fluorides into enamel hydroxyapatite is a fundamental condition for the effectiveness of fluoride prophylaxis.”
Thus, Glenn et al, 1982, demonstrated the relationship between taking fluoride-containing drugs during pregnancy and reducing the intensity of caries in primary (baby) teeth. A number of clinical studies have demonstrated a significant reduction in the intensity of caries in primary and permanent teeth in people living in regions where drinking water is fluoridated (Thylstrup et al, 1982; Newbrun, 1989; Ripa, 1993).
It is known that dynamic processes of de- and remineralization occur on the surface of the tooth, in which the decisive role is played by the concentrations of Ca2+, PO43-, OH- ions in the oral fluid and tooth enamel, as well as the pH value of saliva and interdental space (Borovsky E.V., Leontiev V.K., 1991; Leontiev V.K., 1978, 1980; Pakhomov G.N., 1982).
It was found that the permeability of different layers of intact enamel is not the same. The least permeable is the surface layer, then the subsurface and middle layer (Zero DT, 1999). With the intensification of demineralization processes, an increase in enamel permeability is observed, including the proportion of organic acids (Borovsky E.V., Kuzmina E.M., Nemetskaya T.I., 1986; Vozhov E.A. 1983; Feamhead RW, Kawasaki K. , Inoue K., 1982).
Exposure to organic acids leads to an increase in enamel microspaces, which, in turn, causes a progression of permeability and a further increase in enamel solubility. In addition, the dynamic equilibrium of the processes of de- and remineralization is facilitated by the variability of the mineral composition of the enamel due to the ability of crystals to ion exchange (Morphis TL, Tuomba KJ, Lygidakis NA, 2000; Rosin-Grget K., Lincir I., 2001).
According to M. Neuman (1961), each crystal is covered with a thick hydration layer, allowing ion exchange to occur. The hydration shell indicates that the crystal is electrically charged and may contain foreign ions (Pakhomov G.N., 1982; Curzon MJ, Losee FL, 1977). In addition, the hydration shell is a source of ions participating in exchange and substitution reactions with ions of the surface and deep layers of the hydroxyapatite crystal lattice (Rosin-Gаget K., Lincir I., 2001).
The process of penetration of ions into enamel depends on the ionic radius, activity, chemical properties and concentration of the ions. The state of the hydroxyapatite surface, the charge and concentration of ions on the surface of the hydration layer are also important. Most inorganic ions are smaller in size than the thickness of the hydration layer, so they easily penetrate and accumulate in it (Pakhomov G.N., 1982; Driessens FM, 1982).
It has been established that the process of ion penetration increases with the progression of focal demineralization, which serves as the basis for the remineralization process (Borovsky E.V., Kuzmina E.M., Nemetskaya T.I., 1986; Borovsky E.V., Leontyev V.K., 1991; Pakhomov G.N., 1974; Driessens FM, 1982). The subject of special discussion is the issue of fluorine content in remineralizing solutions.
The introduction of fluoride ions into the composition of remineralizing solutions enhances the remineralization process several times (Tishchenko L. Yu., 2009). Gonzales M. (1998) showed that fluoride increases the rate of incorporation of calcium and phosphorus into enamel. At a fluorine content of 0.1 mg/l, which is a low concentration, apatite precipitates from unstable supersaturated solutions, such as saliva.
In the absence of fluorine, apatite crystals do not precipitate from saturated solutions. In this case, the formation of soluble substances occurs, such as octacalcium phosphate (Knappwost A., 1952; Mount J., 2003).
However, the addition of fluorine to remineralizing solutions leads to its combination with calcium and the formation of an insoluble substance that does not affect the structure of the enamel.
A number of modern experimental data indicate that the key role in the implementation of the kariostatic mechanisms of action of fluorides belongs, first of all, not to the maximum content of fluorapatite in the enamel, but to the presence of ionized fluoride in the environment surrounding the tooth enamel.
In vitro and in situ studies have shown that the key mechanism of the anti-caries effect of fluorides is associated with the regulation of the kinetics of de- and remineralization. According to modern concepts, ionized fluorides provide an anti-caries effect through the following mechanisms:
- obstacle (slowdown) of demineralization of intact enamel;
- influence on the kinetics of remineralization of demineralized enamel;
- optimization of the crystalline composition of enamel (due to calcium fluoride and fluorapatite).
The effect of suppressing enamel demineralization in the presence of ionized fluoride in the environment is of a confirmed dose-dependent nature, which opens up significant opportunities for improving methods of local fluoride prevention by increasing the concentration of ionized fluoride in environments in direct contact with enamel (including in microbial biofilm), compared with biological fluid of the oral cavity.
Stimulation and acceleration of remineralization are associated with the low solubility of fluorapatite, which begins to recover even in a slightly acidic environment earlier than the other phases of enamel apatite. Consequently, during the remineralization that develops after exposure to acid, a redistribution of the mineral phases of the enamel occurs, while the proportion of stable apatites increases, and the proportion of easily soluble calcium phosphate phases decreases (Solovieva A. M., 2009).
Remineralization of tooth enamel is possible only if the function of organic enamel matrices consisting of proteins, glucoproteins, and lipids is preserved (Maksimovsky Yu. M., Zemskova M. I., 1994).
For successful treatment, caries prophylactic and fluoride preparations are used (Table No. 1), which compensate for defects in the crystal lattice, increase the resistance of enamel to acids, and reduce its permeability.
Table No. 1. Composition of fluoride preparations
Classification of caries prophylactic drugs:
I. Fluoride preparations:
A. Fluorine-containing solutions:
- Pro Fluoride M (“VOCO”),
- Fluocal Solute (“Septodont”),
- "Gluftored" ("VladMiVa").
B. Fluoride-containing gels:
- Fluocal Gel ("Septodont"), Fluoridin Gel N 5 ("VOCO"), Pro Fluorid Gelex ("VOCO"), Fluor-Gel ("Blend-a-med"), Oral B Fluor-gel ("Cooper") , Elmex-gele (“Wypert”), PARO AMIN FLUOR ESRO AG (Switzerland), Flor-Opal (Ultradent Product Inc., USA), TOPEX “00: 60” SECOND Sultan (USA), Nupro APF (Dentsply).
B. Fluorine-containing varnishes:
- Fluoridine (“VOCO”), Bifluorid 12 (“VOCO”), ADMIRA PROTECT (“VOCO”), Multifluorid (“VOCO”), Controcar (“Hammacher”), Duraphat (“Woelm”), “Fluor Protector”, “ Composeal", DuraShield® (Sultan chemists inc. USA), white dental varnish Flor-Opal Varnish White (Ultradent Product Inc., USA), Colgate "Duraphat" with sodium fluoride, "Duraphat" (Woelm).
- "Belagel F" ("VladMiVa"), "Ftorlak" (Phthorlacum), "BELAC F" ("VladMiVa", Russia), "Ftorlak" ("Omega-Dent"), "Stomaftor" varnish, "Ftorlak" (transparent ), "Ftorlak" colorless, "RADOGEL F Rainbow-R" (Russia), "PROFILAK" (StomaDent, Russia).
II. Remineralizing drugs:
- Belagel Sa/R (“VladMiVa”),
- enamel sealing liquid (Humanchemie, Germany),
- "Remodent" (Latvia),
- GC Tooth Mousse (Japan),
- MI PASTE PLUS (GC), Dental Resources (USA):
- remineralizing gel with fluoride and calcium,
- fluoride rinse (concentrate).
Indications for the use of caries prophylactic drugs:
- at a therapeutic appointment (for the treatment of caries in the spot stage, multiple dental caries, root caries, hyperesthesia of hard tissues, wedge-shaped defects, for the treatment of restorations after grinding and polishing, with pathological abrasion of teeth, etc.);
- at an orthopedic appointment (after grinding teeth, to protect the living stump of a prepared tooth, when processing the contact surfaces of adjacent teeth, before cementing temporary and permanent crowns on living teeth, when fixing clasp structures, etc.);
- at a periodontal appointment (after curettage of periodontal pockets to protect the necks of teeth, with cervical hyperesthesia, etc.);
- adolescence - in the treatment and prevention of caries in children (preservation of baby teeth, in the presence of pigmented deep fissures of permanent teeth, in the stage of fissure maturation, with immature fissures, etc.);
- at an orthodontic appointment (one month before the start of orthodontic treatment, during fixation and removal of braces and rings);
- restoration of the mineral composition of the enamel after the whitening procedure;
- the final stage after Dentilux or professional oral hygiene;
- pregnancy period.
Effect of fluoride on the metabolism of plaque bacteria
Data on the effect of fluoride compounds on the composition of the microbial flora of dental plaque are contradictory. Thus, some authors point to a decrease in Str. Murans compared to other plaque microorganisms (Loesche WJ, Syed AA, Murray RG, Mellberg JR, 1976). In some studies, no significant changes in the microbial flora were identified (Marsh PD, 1999).
The influence of fluorine on the barrier function of cell membranes of microorganisms was discovered (Edgar WM, Cockburn MA, Jenkins GN, 1981; Wendt LK, Koch G., Birkhed D., 2001). It is assumed that by changing the electrical potential of the cell membrane, the flow of sugars, as well as K+ and PO43- ions into the microbial cell is controlled (Eisenberg AD, Marquis RE, Bender AD, 1980).
A decrease in K+ ions causes depression of acid production by microorganisms (Geddes DAM, Nee SG, 1982; Marsh PD, 1999). When an H+ ion is added to a fluorine ion, a HF molecule is formed, it easily passes through the membrane and dissociates in the cytoplasm into H+ and F- (Luoma H., Luoma AR, Seppa L., 1984). The release of a proton helps reduce the pH of the cytoplasm, and fluorine ion can directly inhibit the process of glycolysis and the synthesis of acids by microorganisms, which ultimately leads to bacteriolysis (Broukal Z., Zajicek O., 1974).
In addition, some authors believe that fluorides can influence the adhesion of dental jack bacteria to tooth enamel by disrupting the formation of extracellular polysaccharides, a substance that plays a major role in bacterial adhesion (Brudevold F., Naujoks R., 1978; Ferreti GA, Tanzer JM , Tinanoff N., 1982). Fluorides also slow down the formation of lipoteichonic acid, negatively affecting the ability of bacteria to adhere to the tooth surface.
Fluoride affects various stages of the metabolism of plaque bacteria, especially carbohydrate metabolism and, in particular, the processes of glycolysis. In this case, the activity of the enolase enzyme is inhibited. Under the influence of enolase, phosphoenolglycerate is converted to phosphoenolpyruvate. Enolase activity depends on the availability of free magnesium. Fluoride ions bind to magnesium, which leads to inhibition of enolase activity.
Fluorine also slows down the transport of glucose into bacterial cells. Penetration of glucose into the streptococcal cell can occur in two ways. Through the phosphoenolpyruvate phosphotransferase (PEP-PTP) system, glucose-6-P is formed and penetrates into the cell.
In this case, activated phosphate is formed from phosphoenolpyruvate. Fluorine has an indirect inhibitory effect on the intracellular synthesis of polysaccharides, since due to the cessation of the functioning of the FEP-PTF mechanism, the formation of glucose necessary for the synthesis of polysaccharides slows down or this process does not occur. Fluorine does not affect the breakdown of intracellular reserve carbohydrates.
As already mentioned, fluorides inhibit the formation of phosphoenolpyruvate. At a low pH value in the extracellular space, fluoride ions combine with a proton and penetrate into the cell in the form of HF. The value of the proton gradient decreases, and as a result, the transport flux of glucose decreases. Due to the dissociation of HF in the intracellular space, the pH value decreases. Since glycolytic enzymes are most active in an alkaline environment, a decrease in pH inhibits bacterial metabolism (Helwig E., Klimek J., Attin T., 1999).
Thus, under normal physiological conditions, fluoride-containing compounds have a slight effect on plaque bacteria, but by affecting the metabolic processes of plaque, they can change its microbial composition.
The mechanism of the anti-caries effect of fluoride
Under the best conditions, that is, when a person receives the optimal amount of fluoride throughout his life, the following links in the complex mechanism of the anti-caries effect of fluorides can be realized.
The first link is during the formation of the organic matrix and its primary mineralization. Optimal fluoride supply during this period promotes matrix synthesis, crystal formation and the mineralization process.
The second link is after the end of the activity of ameloblasts in the pre-eruptive stage, which lasts several years, when the crowns of the teeth are still chemically unstable. Due to the relatively easy replacement of hydroxyl with fluoride ion, the enamel crystal lattice is enriched with fluorapatite with the formation of structures more resistant to physical, chemical and biological influences. Therefore, it is believed that the second link of the anti-caries effect of fluorides is even more important:
Ca10(PO4)6(OH)2 + F– à Ca10(PO4)6F(OH) + (OH)– (1)
As a result of the isomeric substitution reaction from hydroxyapatite, hydroxyfluorapatite is obtained. This compound is significantly more resistant to dissolution than hydroxyapatite. When fluorine replaces even one of the 50 hydroxyl groups, the solubility of enamel decreases sharply. However, when hydroxyapatite is exposed to high concentrations of fluorine, the reaction follows a different type:
Ca10(PO4)6(OH)2 + 20F– à 10СаF2 + 6PO43– + 2(OH)– (2)
As a result of this reaction, calcium fluoride is formed, a practically insoluble compound that quickly disappears from the tooth surface as a result of leaching. This reaction during fluorination is undesirable, and therefore high concentrations of fluorides should not be used, especially in acidic solutions.
The third link is in the post-eruptive period, when ion exchange slows down significantly; at this stage, fluoride-containing water and somewhat fluoride-enriched saliva, washing the tooth crown, maintain the barrier properties of the surface layers of enamel and, possibly, contribute to its remineralization (Loshakova L. Yu., 2011).
The mechanism of action of fluoride preparations on tooth enamel
The main principle of remineralization is the compensation of the loss of mineral elements in demineralized solid tissues, which is ensured by increasing the permeability of the latter to ions and drug molecules.
During remineralization therapy, enamel solubility changes in two phases: first, there is a rapid increase in enamel solubility with a significant decrease in excess Ca2+ release. After six months, the solubility of the enamel decreases again and the processes of de- and remineralization reach equilibrium (Maksimovskaya L.N., 2002).
Rolla G. (1988) studied the effect of calcium fluoride formed on the demineralized surface of tooth enamel. It was found that CaF2 helps to increase enamel resistance by blocking diffusion pathways during acid attack.
However, due to their rather large size, calcium fluoride molecules are unable to remain on the tooth surface for a long time and, accordingly, prevent demineralization for a long time and enhance remineralization (Rosalen PL, Pearson SK, Bowen WL, 1996).
The mechanism of local action of fluoride preparations on tooth enamel is their inclusion in the crystal lattice of the enamel. In this case, hydroxyl or another less active ion is replaced by F—, which leads to the formation of a substance more resistant to acid dissolution (Knappwost A., 1978; Tishchenko L. Yu., 2009). It has been established that after application of a fluoride solution acidified with phosphate, fluoride is adsorbed in the form of CaF2 crystals on the organic substance of the enamel.
When exposed to fluorides in an amount of 0.1-1.0 mg/l, calcium phosphate complexes and a thin layer of fluorapatite are formed on the enamel surface (Ganss C., 2001). Exposure to solutions containing fluorides in amounts below 0.5 mg/l leads to the formation of mainly fluorapatite, accelerating remineralization. Solutions containing fluorides more than 0.5 mg/l lead to the formation of fluorite on the surface of teeth (Tishchenko L. Yu., 2009).
When the enamel surface was exposed to combinations of 8% solutions of sodium fluoride and tin fluoride with sodium acid phosphate (NaH2PO4), a high anti-caries effectiveness of the combination of tin fluoride with acid phosphate was noted. Abrahams L. et al. (Bratthall D., 1995) used a system containing calcium fluoride to remineralize enamel, which serves as a source of fluoride and, together with calcium and salivary phosphates, provides the thermodynamic driving forces for the formation of fluorapatite and hydroxyfluorapatite.
Thus, F. Brudevold, RF Naujok (1978) indicate that when fluorides are used in low concentrations, calcium fluoride and fluorapatite are formed, while the use of high concentrations of fluorides leads mainly to the formation of a soluble octocalcium phosphate compound, which is unable to significantly influence the remineralization process.
However, according to the author, the positive effect of exposure to fluorides is short-term, decreasing as fluorine ions leave the enamel (Brudevold F., Naujoks R., 1978). Numerous studies have confirmed that the process of fluorapatite formation is more intense when fluorides are used in low concentrations (Borovsky E.V., Maksimovskaya L.N., Kolesnik A.G., 1987; Leontyev V.K., 1980; Duschner H., Gotz FL, Ogaard B., 1997; Gibbs CD, Atherton SE, Huntington E., 1995).
Jenkins GN (1988) believes that fluorine ions, replacing the OH- group or carbonate included in the apatite structure, affect the formation of crystals, increase their size, and also contribute to the remineralization process by precipitating apatites from supersaturated solutions. There is information in the literature confirming this (Stookey G. K., 1998; Strohmmgei L., Brambilla E., 2001).
Larsen M., Fejersckov O., Tachos B. (1981) found that constant maintenance of even slightly increased fluoride concentrations in the oral cavity helps reduce solubility and promotes the remineralization process.
Changes in enamel with a single increase in fluoride concentration are too small, but if they are maintained for a long period of time, this provides protection against the release of calcium and phosphorus from the surface layer of enamel, and also promotes the entry of fluoride ions into the enamel structure and the formation of fluorapatite. This effect was achieved with daily use of fluoride pastes and rinses (Kuzmina E. M., 2001; Nedoseko V. B., Lomiashvili L. M., 1995).
There is also information in the literature that the use of fluoride-containing preparations promotes the formation of a protective covering layer of mineral components on the surface of the enamel, as well as in the pores of loosened enamel during the initial stages of caries.
This protective reaction is disrupted if the rate of apatite dissolution exceeds the rate of formation of the protective layer. Fluorine-containing preparations help increase the rate of formation of a protective mineral layer (Feamhead RW, Kawasaki K., Inoue K., 1982; Knappwost A., 1978, 1993; Wen HB, Cui FZ, Chen XQ, Wang G., 1995).
The pronounced therapeutic effect of fluorides is explained not only by the fact that it helps to increase the accumulation of calcium and phosphorus ions in the surface layers of enamel, but also by the fact that in the absence of fluorine, apatite crystals do not precipitate from saturated solutions, but form soluble compounds such as octacalcium phosphate. Thus, the combination of the use of remineralizing compounds and fluorine significantly increases the effect of their impact (Strohmmgei L., Brambilla E., 2001).
However, when studying tooth enamel after applications of sodium fluoride solutions, a decrease in the penetration of radioactive calcium into the deep layers of enamel was noted compared to the surface layer.
This indicates that the enamel acts as an ionic membrane, and the density of the negative charge on the crystal surface increases after local treatment with fluorine, which creates a higher cation specificity of the enamel (Christofersen J., Christofersen MR, Arends J., Leonardsen E., 1995 ; Featherstone JD, 2000; Warren DP, Chan JT, 1997).
Fluorides affect metabolic processes in tooth enamel. During the interaction of fluorides with enamel, apatite crystals are deposited on the surface of the so-called protective layer, which explains the decrease in permeability and the intensification of remineralization processes.
Along with inorganic fluorine compounds, its organic compounds, amino fluorides, have recently found use. According to many authors, they are more intensively incorporated into the enamel, increasing its resistance to acid dissolution, which is associated with the formation of calcium fluoride (Leus P. A., Smirnova T. A., Pashinina E. O., 1980; Khomenko L., Kononovich E., 1997).
The idea of using amino fluoride as a topical agent is associated with the remineralizing effect of fluorine and the physicochemical properties of protecting aliphatic amines. Aminofluoride is able to protect enamel from acids not only due to the formation of CaF2, but also due to the precipitation of amine additives (Kolesnik A. G., Pilat E. L., 1989; Brambilla E., Tosilli A., Felconi A., Gagliani M. , 1997; Faller RV, 1995).
A number of authors (Smirnova T. A., 1984; Jungo Markus, 2001) used applications of 1% sodium fluoride gel for remineralization. They believe that the mechanism of preventive action of fluoride gel is the inhibition of lactate and acetate by plaque bacteria.
Most researchers indicate high efficiency when using fluorovarnishes and fluorogels on smooth surfaces (Bakhmudov B. R., 1994; Kuzmina E. M., Smirnova T. A., 2001; Maslak E. E., Kazantseva I. A., Fursik T.I., Ogonyan V.R., 1997).
The use of fluorides in the form of gels and varnishes has a number of advantages. They have pronounced adhesion to enamel, are easy to apply and can remain on the enamel for up to 24 hours (Kolesnik A.G., Pilat E.L., 1989; Kuzmina E.M., 2001; Attin T., Hartmann O., Hilgers RD , Hellwig E., 1995).
However, the WHO expert report (1995) indicates that the release of fluorides is “explosive” and short-term in nature. Special membranes are also used to slowly release fluorides. The duration of fluoride release can be 30-80 days. These methods are likely to play an important role in the prevention and treatment of caries in the future, but at present there are no comprehensive data from clinical trials (Castioni N., Beahni PC, Gurny R., 1998).
Despite various approaches to the use of fluorides in the oral cavity, numerous data indicate that the effect of their exposure does not exceed 45-56%. Analyzing the literature data, we can conclude that various fluoride compounds are widely used for the prevention of dental caries. However, the effectiveness of simple fluorides is short-term and requires long-term and frequent use to achieve a pronounced preventive effect.
An excess of carbohydrate foods and simple sugars leads to an increase in the viscosity of saliva, which significantly reduces its mineralizing properties. According to some authors, viscous saliva prevents the washing away of fermentation acids from the points where food residues are retained on the teeth, which causes an increase in the processes of demineralization of the enamel.
In addition, with a carbohydrate load, there was a decrease in the functional activity of the salivary glands, a change in the physicochemical properties of saliva: a decrease in the flow rate, a change in its qualitative and quantitative composition (Kruglova L.N., Nedoseko V.B., Ter-Nikogosova L., 1988 ; Suntsov V. G., 1987; Chekmezova I. V., 1983).
The influence of oral fluid pH on enamel mineralization
A significant role in reducing the mineralizing function of saliva is played by changes in pH. It is believed that the average pH value of saliva is 6.5-7.5, that is, it is neutral. A sharp decrease in pH is observed with a carbohydrate load (Borovsky E.V., Burdina O.V., 1991; Leontyev V.K., Petrovich Yu.A., Kruglova L.N., 1997; Leus P.A., Lebedeva G. K., 1981).
When studying the effect of sucrose on the composition of the properties of mixed saliva in children, an increase in viscosity was noted, as well as a decrease in the pH of the oral fluid (Redinova T. L., 1989; Tokueva L. I., 1985).
One of the main factors that destabilizes the pH of oral fluid is the acid-forming ability of dental plaque microorganisms (Balashova E. I., 1987; Levitsky A. P., Mizina I. K., 1987; Leus P. A., Lebedeva G. K. , 1981; Redinova T. L., 1989; Mascarenhas AK, Moursi AM, 2001).
In studies on the effect of changes in the pH of saliva on its composition, it was proven that when the pH decreased below 5, the concentration of hydrogen ions decreased by 10 times, the degree of saturation with hydroxyapatite by 8.3 times. Moreover, according to the authors, pH 6–6.62 is critical. Saliva passes from the state of a mineralizing liquid to a demineralizing state (Borovsky E.V., Leontyev V.K., 1991; Borovsky E.V., Leontyev V.K., Maksimovskaya L.N., 1984; Gron P., 1983; Larsen M J., 1991).
On the contrary, an increase in pH increases the mineralizing properties of the oral fluid due to an increase in the degree of supersaturation with Ca2+, HPO42-, F- ions. This promotes the formation of tartar and partly explains the resistance of teeth to caries in the area of its formation (Murray II, 1986; Navarro M., 2001).
Van Houte J., Lopman J., Kent R. (1996) consider changes in local pH to be the main cause of focal demineralization (Woltgens JH, Bervouets TJ, Witjes F., Houwink B., 1981). However, according to other authors, minor fluctuations in local pH cannot greatly change the pH of the oral fluid as a whole (Kanellis MJ, 2000).
A short-term decrease in local pH at the site of exposure to organic acids of dental plaque on tooth enamel is quickly restored due to the consumption of ions contained in the oral fluid: Ca2+, HPO42-, F-, OH-. Thus, partial precipitation of the lost ions occurs and the local pH value is restored.
As noted by many authors (Leontiev V.K., 1983; Leontiev V.K., Chekmezova I.V., Shvyrnogov V.Z., 1983; Morphis TL, Tuomba KJ, Lygidakis NA, 2000), the main mechanism for maintaining the constancy of dental tissues in the oral cavity is the oversaturation of the oral fluid with Ca2+, HPO42-, F- ions, in other words, hydroxyapatite or fluorapatite.
A. Knappwost (1993) states that the surface layer of enamel is covered predominantly with Ca2+ and PO43– ions, which, as a result of hydrolysis in the intermediate stage, forms CaHPO4, and in the final hydroxyapatite Ca10(PO4)6∙(OH)2. Hydroxyapatite, formed on the surface of the enamel, due to its low solubility and stability, can play a protective role.
The progression of caries, therefore, occurs when the rate of spread of the carious lesion is higher than the rate of formation of hydroxyapatite in the covering layer (Knappwost A., 1993).
It has been proven that the caries-preventive effect of molybdenum and aluminum is due to the suppression of the release of calcium from the enamel when exposed to acids and the stimulation of the inclusion of fluorine in the hydroxyapatite lattice (Kleder CJ, Putt MS, 1984), in addition, sodium chloride helps to increase the effectiveness of fluoride ions, and zinc ions suppress the activity bacteria (RF Patent No. 2157174 dated March 29, 1999).
The main result of the cariesostatic effect of fluoride is the suppression of the demineralization process or the activation of remineralization of hard dental tissues. In the oral cavity, the surface of the tooth is constantly exposed to various influences.
Immediately after eruption, the surface of the tooth is covered with greater or less density by various microorganisms, the metabolic activity of which, in the presence of an appropriate substrate, can lead to a decrease in the pH level and, as a result, to the induction of the demineralization process. Periods of demineralization can be replaced by periods of remineralization if, as a result of the transfer by saliva (clearance) of metabolic products of microorganisms and substrate, the pH value increases.
The remineralization effect is the most important of them. Fluoride ions penetrating into the thickness of the enamel and into its surface lead to the strengthening of the enamel, which not only becomes more resistant to carious attack, but also acquires the ability to remineralize or heal the initial manifestations of caries caused by the acids of cariogenic microorganisms. The fluoride ions necessary for remineralization are provided both by fluoridated water and various caries-preventive drugs, for example fluoride drugs (Table No. 1).
Thus, summarizing the data available in the literature, we can conclude that an increase in the viscosity of saliva and changes in the pH of the oral fluid in general, as well as sharp decreases in local pH at the site of exposure of the microbial flora directly to the tooth enamel lead to a decrease in its saturation with hydroxyapatite, which disrupts the processes re- and demineralization towards strengthening the latter.
Changes in enamel at the ultrastructural level lead to the formation of a lesion, diagnosed visually as caries in the white spot stage.
Based on all of the above, we can conclude that the use of caries prophylactic drugs with an optimally selected composition allows for the most effective prevention of caries both at the individual and social levels, and the use of systemic methods of fluoride prevention should be prescribed additionally, taking into account a number of features, and comply with internationally accepted recommendations for the use of fluorides.
- Zavyalova T. G. Prevention and treatment of caries in the white spot stage using the method of deep fluoridation [Text]: abstract. dis. for the job application scientist step. Ph.D. honey. Sciences / T. G. Zavyalova. - M., 2003. - 19 p.
- Kiselnikova L.P. Prospects for local use of fluorides in clinical dentistry [Text] / L.P. Kiselnikova // Maestro of Dentistry. — 2007, No. 2 (26). - P. 18-22.
- Kolesnik A.G., Sakharova E.B. Means for the prevention of dental caries // Medical assistance. - M., 1995. - No. 6. - P. 43-45.
- Lobovkina L. A. Prevention in therapeutic dentistry is profitable! L. A. Lobovkina, A. M. Romanov [Text] / New in dentistry. - 2007, No. 6. - P. 46-49.
- Loshakova L. Yu. Course of lectures on current issues in pediatric dentistry: textbook / Loshakova L. Yu., Pylkov A. I.; GOU VPO KemSMA Roszdrav. - Kemerovo: KemGMA, 2011. - 187 p.
- Markskors D. Preparation of teeth for fixation of crowns (chapters from the monograph) / D. Markskors, R. Markskors // New in dentistry. For dental technicians. - 2003, No. 1. - P. 73-76.
- Pakhomov G.N. Primary prevention in dentistry. - M., 1982. - 240 p.
- Solovyova A. M. Fundamentals of the anti-carious effect of fluorides [Text] / A. M. Solovyova // Institute of Dentistry. - 2009, No. 45. - 32-35 p.
- Tishchenko L. Yu. Clinical assessment and increase in the resistance of enamel and dentin in hyperesthesia of hard dental tissues [Text]: abstract. dis. for the job application scientist step. Ph.D. honey. Sciences / L. Yu. Tishchenko - Stavropol, 2009. - 22 p.
- Fleischer G. M. “Dentilux” - professional oral hygiene [Text] / G. M. Fleischer // Dental South. - 2011, No. 10. — P. 48-51.
- Helwig E., Klimek J., Attin T. Therapeutic dentistry. Ed. prof. AM Politun, prof. N. I. Smolyar. Per. with him. - Lvov: GalDent, 1999. - 409 p.
- Knappwost A. Eine dringende Aufgade fur die zahnärztliche Praxis Tiefenfluoridierung durch mineralsche Schmelzvesiegelung // Dtsch. Zahnarztl. Ztschr. - 1993, No. 8. - R. 20-23.
When can it be used?
The use of Belak F is recommended for:
- initial caries;
- prevention of dental caries in children and adolescents with low enamel resistance;
- wedge-shaped defects;
- increased sensitivity of teeth;
- traumatic damage to enamel;
- erosion on the tooth surface;
- increased tooth wear;
- initial stages of gum recession;
- carrying out professional oral hygiene.
Recommendations
Manipulations for the use of Belak F are recommended to be performed only at a dentist’s appointment, where everything necessary for the procedure is available. The drug is not intended for use at home! In addition to everything, it must be emphasized that after finishing it is worth refusing water for 2 hours and, accordingly, food for 12, so as not to damage the formed protective layer. The most ideal time to do this is before bed, but it is quite difficult to actually implement this at the reception.
Belak F for teeth is produced not only for the CIS markets, but also for other countries, as a cheaper analogue of Western fluoridated varnishes.
Like any other medication, everyone has their own perception, for example, doctors strongly do not recommend using it for: fluoride intoxication (fluorosis), increased bleeding of the gums and individual intolerance to the components of the composition. But if we talk about when it can and even should be used, then the wishes are as follows: if you have the initial stage of caries, increased tooth sensitivity, erosion, wedge-shaped defects, gum recession and simply for prevention.
Method of use
Stages of applying varnish:
- cleansing all tooth surfaces with a brush and toothpaste that does not contain fluoride;
- insulation of the working area with cotton rollers;
- drying the tooth with air;
- Using an applicator, the varnish is applied to the surface of the tooth and distributed evenly;
- after all the doctor’s manipulations, the patient should sit with his mouth open for 4-5 minutes until the drug dries completely;
- then all the videos are removed and recommendations are given.
The patient should not brush his teeth or eat solid food for 12 hours after applying the product. You should refuse water for 2 hours.
For preventive purposes, Belak F is recommended to be used 2-3 times a year. When treating caries, fluoride varnish is applied according to a certain course. The patient receives 3-4 applications every 3 days.
Before use, the bottle must be shaken to restore the homogeneity of the material.
The drug is stored in a bottle with a tightly closed lid in a cool place. Over time, delamination of the drug may occur, but this does not affect its performance.
Increased tooth sensitivity can cause very severe discomfort. In such a situation, Belak F becomes simply irreplaceable. It relieves all unpleasant sensations after the first use. Of course, existing enamel defects will not change in appearance, but they will become much less of a concern.
The only disadvantage of using varnish at home is the inconvenience of applying it. Drying the tooth surface completely is not an easy task. Saliva constantly flows and interferes with the use of the drug. But, if you get used to it, there should be no problems with use.
It is better to discuss the frequency of use with your dentist in advance to avoid overdose.
Instructions for use
First you need to remove plaque with toothpaste or tooth powder that does not contain fluoride. Or thoroughly clean the surfaces of the teeth with a brush and water.
- Isolate the gums from the working sides with cotton swabs so that Belak F fluoride varnish does not get on the oral mucosa.
- Dry your teeth with air to remove any remaining saliva or moisture.
- Using an applicator stick, apply and spread the product evenly.
- Do not close your mouth for 5-7 minutes until the medicine dries and forms a protective layer.
- After 12 hours, remove the film with a toothbrush dipped in water.
Belak F for teeth
The drug most commonly found in trade is white and transparent, but you can also find it in yellow. There is no significant difference in them, only in the ease of application, since yellow stands out due to its color and is easy to detect on the teeth, unlike colorless ones. Also included are applicators that are quite convenient to apply Belak F without touching the mucous membrane.
Cost and analogues
The price of Belak F is about 120-150 rubles per bottle.
You can find a large number of similar products on the dental materials market:
- Bifluorid 12, manufactured by VOCO;
- Fluoride varnish from Omega-Dent;
- Tooth Mousse gel from GC;
- manufacturer Vladmiva produces the drug in the form of Belagel gel;
- Fluocal from the manufacturer Septodont.