Composition and release form
| Soluble tablets | 1 table |
| amoxicillin (as amoxicillin trihydrate) | 125 mg |
| 250 mg | |
| 500 mg | |
| 1000 mg | |
| excipients: dispersible cellulose; MCC; crospovidone; vanillin; tangerine flavor; lemon flavoring; saccharin; magnesium stearate |
5 pcs in blister; there are 4 blisters in a box (125, 250, 500, 1000 mg) or 7 pcs in a blister; There are 2 blisters in a box (125 mg).
Flemoxin Solutab
Flemoxin solutab
® (lat.
Flemoxin Solutab
®) is an antibiotic of the penicillin class. The active ingredient is amoxicillin.
Dosage forms of flemoxin solyutab
Flemoxin Solutab is available in the form of dispersible (soluble) tablets containing 125, 250, 500 or 1000 mg of amoxicillin (as amoxicillin trihydrate). Tablets are white to light yellow in color, oval in shape, from white to light yellow in color with the company logo and digital designation on one side and a score dividing the tablet in half on the other side. The number on the tablet indicates the content of amoxicillin:
- “231” - tablet contains 125 mg of amoxicillin
- “232” - tablet contains 250 mg of amoxicillin
- “234” - tablet contains 500 mg of amoxicillin
- “236” - tablet contains 1000 mg of amoxicillin
Indications for use of flemoxin solyutab
Flemoxin solutab is used in the treatment of infectious and inflammatory diseases caused by microbes sensitive to flemoxin solutab, including:
- infectious diseases of the respiratory system
- infectious diseases of the genitourinary system
- infectious diseases of the skin and soft tissues
- infectious diseases of the gastrointestinal tract, including Helicobacter pylori
-associated gastric and duodenal ulcers, atrophic gastritis, MAL Tomas.
Flemoxin solutab in gastroenterology
Gastroenterologists most often use flemoxin solutab as one of the antibiotics as part of complex therapy for the eradication of Helicobacter pylori
.
Flemoxin solutab is not used in the eradication of Helicobacter pylori
outside of special regimens, without drugs that reduce gastric acidity.
The dosage and procedure for taking Flemoxin Solutab depends on the eradication “scheme” used (see the article “Amoxicillin” or “Standards for the diagnosis and treatment of acid-dependent and Helicobacter pylori-associated diseases (fourth Moscow agreement)”) Flemoxin Solutab is also not used in any form for treatment of gastric and duodenal ulcers or gastritis in the absence of Helicobacter pylori
.
Method of administration of Flemoxin Solutab and dose
Flemoxin solutab is taken orally, before, during or after meals, swallowed whole, or divided into parts or chewed, washed down with a glass of water or diluted in water to form a syrup (20 ml) or suspension (100 ml).
For adults and children over 10 years of age, for mild to moderate infections - 500–750 mg 2 times a day or 375–500 mg 3 times a day.
Children from 3 to 10 years old - 375 mg 2 times a day or 250 mg 3 times a day; from 1 year to 3 years - 250 mg 2 times a day or 125 mg 3 times a day. The daily dose of Flemoxin Solutab for children under 1 year of age is 30–60 mg per kg of weight, divided into 2–3 doses.
When treating severe infections and hard-to-reach lesions, it is advisable to take flemoxin solutab three times a day.
For chronic diseases, relapses, severe infections: adults - 0.75–1 g 3 times a day, children - up to 60 mg per kg per day; The dose calculated in this way is divided into 3 doses.
For acute uncomplicated gonorrhea - 3 g once, in combination with 1 g probenecid.
For mild to moderate infections, treatment with flemoxin solutab is 5–7 days, for infections caused by Streptococcus pyogenes
, - at least 10 days.
When treating chronic diseases and severe infections, the dose of Flemoxin Solutab should be determined by the clinical picture of the disease. Flemoxin solutab is continued for two days after the symptoms of the disease disappear.
When creatinine clearance is less than 10 ml per minute, the dose of Flemoxin Solutab is reduced by 15–50%.
Professional medical articles regarding the use of flemoxin solutab in the eradication of Helicobacter pylori
- Potapov A.S., Pakhomovskaya N.L., Dublina E.S. and others. Evaluation of the effectiveness and safety of triple eradication therapy for helicobacteriosis in children with the drugs helol, de-nol and flemoxin solutab // Almanac of Clinical Medicine. – 2006. – volume XIV. - With. 87–94.
- Samsonov A.A., Maev I.V., Ovchinnikova N.I., Shakh Yu.S., Podgorbunskikh E.I. The effectiveness of using colloidal bismuth subcitrate in Helicobacter pylori eradication therapy regimens for duodenal ulcer // RZHGGK. 2004. No. 4. pp. 30–35.
On the website gastroscan.ru in the literature catalog there is a section “Antibiotics used in the treatment of gastrointestinal diseases”, containing articles on the use of antimicrobial agents in the treatment of diseases of the digestive tract.
Other medicines containing the active ingredient amoxicillin
Amoxicillin, Amoxicillin, Amoxicillin capsules 0.25 g, Amoxicillin DS, Amoxicillin sodium sterile, Amoxicillin Sandoz, Amoxicillin-ratiopharm, Amoxicillin-ratiopharm 250 TC, Amoxicillin powder for suspension 5 g, Amoxicillin tablets, Amoxicillin trihydrate, Amoxicillin trihydrate (P urimox) , Amosin Gonoform, Gramox-D, Grunamox, Danemox, Ospamox, Hiconcil, Ecobol.
general information
According to the pharmacological index, Flemoxin Solutab belongs to the “Penicillin” group, according to the ATC - to the “Broad-spectrum Penicillin” group and has the code “J01CA04 Amoxicillin”.
Flemoxin Solutab is a generic version of amoxicillin and therefore information about its medicinal properties, including: indications for use, dosage regimen, list of microorganisms against which Flemoxin Solutab is active, Flemoxin Solutab in Helicobacter pylori
, antibiotoresistance
of Helicobacter pylori
to flemoxin solutab (amoxicillin), preservation of intestinal microflora during therapy with flemoxin solutab, pharmacokinetics of flemoxin solutab, interaction of flemoxin solutab with other drugs, contraindications when taking flemoxin solutab - see the article “Amoxicillin”.
The manufacturer of Flemoxin Solutab is Astellas Pharma Europe B.V. (Astellas Pharma Europe BV), Holland.
Instructions from the manufacturer (pdf): “Instructions (information for specialists) on the medical use of the drug Flemoxin Solutab.”
Flemoxin solutab has contraindications and application features; before starting therapy, consultation with a specialist is necessary.
Back to section
Pharmacodynamics
Active against such gram-positive and gram-negative microorganisms as Streptococcus pyogenes, Streptococcus pneumonia, Clostridium tetani, C.welchii, Neisseria gonorrhoeae, Neisseria meningitidis, Staphylococcus aureus (not producing beta-lactamase), Bacillus anthracis, Listeria monocytogenes, Helicobacter pylori. Less active against Enterococcus faecalis, Escherichia coli, Proteus mirabilis, Salmonella typhi, Shigella sonnei, Vibrio cholerae. Not active against microorganisms producing beta-lactamases, Pseudomonas spp., indole-positive Proteus spp., Serratia spp., Enterobacter spp.
Pharmacokinetics
Suction
After oral administration, it is absorbed quickly and almost completely (about 93%), acid-stable. After oral administration at a dose of 500 mg, the Cmax of the active substance in plasma is 5 mcg/ml and is observed in the blood plasma after 2 hours. When the dose of the drug is increased or decreased by 2 times, Cmax in the blood plasma also changes by 2 times. Food intake has virtually no effect on the absorption of the drug.
Distribution
Plasma protein binding is about 20%. Amoxicillin penetrates well into mucous membranes, bone tissue, intraocular fluid and sputum in therapeutically effective concentrations. The concentration of amoxicillin in bile exceeds its concentration in blood plasma by 2–4 times. In amniotic fluid and umbilical vessels, the concentration of amoxicillin is 25–30% of its level in the blood plasma of a pregnant woman.
It penetrates poorly through the BBB, but during inflammation of the meninges, the concentration in the cerebrospinal fluid is about 20% of the concentration in the blood plasma.
Metabolism
Partially metabolized, most of its metabolites are inactive against microorganisms.
Removal
It is eliminated primarily by the kidneys, about 80% by tubular excretion, 20% by glomerular filtration. In the absence of renal dysfunction, T1/2 is 1–1.5 hours. In premature infants, newborns and children under 6 months—3–4 hours.
Pharmacokinetics in special clinical cases.
T1/2 of amoxicillin does not change with impaired liver function.
If renal function is impaired (Cl creatinine ≤15 ml/min), T1/2 of amoxicillin may increase and reaches 8.5 hours in anuria.
Flemoxin solutab 1000 mg 20 pcs. dispersible tablets
pharmachologic effect
Semi-synthetic antibiotic penicillin.
Composition and release form Flemoxin solutab 1000 mg 20 pcs. dispersible tablets
Tablets - 1 tablet:
- active ingredient: amoxicillin (in the form of amoxicillin trihydrate) - 125 (145.1) mg, 250 (291.4) mg, 500 (582.8) mg, 1000 (1165.5) mg;
- excipients: dispersible cellulose, microcrystalline cellulose, crospovidone, vanillin, tangerine flavor, lemon flavor, saccharin, magnesium stearate.
Dispersible tablets 125 mg - 5 or 7 tablets in a blister made of polyvinyl chloride film and aluminum foil. 4 or 2 blisters along with instructions for use are placed in a cardboard box.
Dispersible tablets 250 mg, 500 mg and 1000 mg - 5 tablets each in a blister made of polyvinyl chloride film and aluminum foil. 4 blisters along with instructions for use are placed in a cardboard box.
Description of the dosage form
Tablets are white to light yellow in color, oval in shape, with the company logo and digital designation on one side and a line dividing the tablet in half on the other side.
Numerical designation: 125 mg tablets - “231”, 250 mg tablets - “232”, 500 mg tablets - “234”, 1000 mg tablets - “236”.
Characteristic
A bactericidal, acid-resistant, broad-spectrum antibiotic from the group of semisynthetic penicillins.
Directions for use and doses
Flemoxin Solutab® is taken orally regardless of food intake.
Immediately before use, the tablet should be diluted in water (at least 50 ml) and mixed thoroughly. The resulting mixture, which has a light fruity taste, should be taken immediately after preparation.
Doses
When choosing the dose of Flemoxin Solutab® for the treatment of certain infections, the following factors should be considered:
- suspected pathogens and their likely sensitivity to antibacterial drugs;
- severity and location of infection;
- the patient's age, weight, and renal function as described below.
The duration of treatment depends on the type of infection and the patient's clinical response and should be as short as possible. Some infections require longer treatment.
Adults and children ≥40 kg
| Indications for use * | Dose* |
| Acute bacterial sinusitis | 250-500 mg every 8 hours or 750 mg-1 g every 12 hours. For severe infections, 750 mg-1 g every 8 hours. For the treatment of acute cystitis, it is possible to take 3 g twice a day. |
| Asymptomatic bacteriuria during pregnancy | |
| Acute pyelonephritis | |
| Dental abscess with inflammation of the subcutaneous tissue | |
| Acute cystitis | |
| Acute otitis media | 500 mg every 8 hours or 750 mg-1 g every 12 hours. For severe infections, 750 mg-1 g every 8 hours for 10 days. |
| Acute streptococcal tonsillitis and pharyngitis | |
| Exacerbation of chronic bronchitis | |
| Community-acquired pneumonia | 500 mg-1 g every 8 hours. |
| Typhoid and paratyphoid | 500 mg-2 g every 8 hours. |
| Infections of prosthetic joints | 500 mg-1 g every 8 hours. |
| Prevention of bacterial endocarditis during surgical procedures in the oral cavity and upper respiratory tract. | 2 g orally, single dose 30-60 minutes before surgical procedure in the oral cavity and upper respiratory tract. |
| Helicobacter pylori eradication | 750 mg-1 g twice daily in combination with a proton pump inhibitor (eg, omeprazole) and another antibiotic (eg, clarithromycin, metronidazole) for 7 days. |
| Lyme disease | Early stage: 500 mg-1 g every 8 hours, maximum daily dose of 4 g, divided into divided doses, for 14 days (range 10 to 21 days). Late stage (systemic infection): 500 mg-2 g every 8 hours, maximum daily dose of 6 g, divided into divided doses, for 10-30 days. |
| *Official clinical guidelines for each indication should be taken into account. | |
Children weighing ≥40 kg
Children weighing more than 40 kg should take the dose recommended for adults.
Overweight children
| Indications for use * | Dose* |
| Acute bacterial sinusitis | 20-90 mg/kg/day, divided into 2-3 doses**. |
| Acute otitis media | |
| Community-acquired pneumonia | |
| Acute cystitis | |
| Acute pyelonephritis | |
| Dental abscess with inflammation of the subcutaneous tissue | |
| Acute streptococcal tonsillitis and pharyngitis | 40-90 mg/kg/day, divided into 2-3 doses**. |
| Typhoid and paratyphoid | 100 mg/kg/day, divided into 3 doses. |
| Prevention of bacterial endocarditis during surgical procedures in the oral cavity and upper respiratory tract | 50 mg/kg orally, a single dose 30-60 minutes before oral and upper respiratory tract surgery. |
| Lyme disease | Early stage: 25-50 mg/kg/day, divided into 3 doses, for 10-21 days. Late stage (systemic infection): 100 mg/kg/day, divided into 3 doses, for 10-30 days. |
| * The official guidelines for each indication should be taken into account. **Only when amoxicillin is prescribed in the upper dose range should twice daily use be considered. | |
Dosage regimen for certain categories of patients
Elderly patients
No dose adjustment is required.
Patients with kidney failure
| Glomerular filtration rate (ml/min.) | Adults and children ≥ 40 kg | Children |
| Over 30 | No adjustment needed | No adjustment needed |
| 10-30 | Maximum 500 mg twice daily | 15 mg/kg twice daily (maximum 500 mg twice daily) |
| Less than 10 | Maximum 500 mg per day | 15 mg/kg once daily (maximum 500 mg daily) |
| * In most cases, parenteral treatment is preferred. | ||
Patients receiving hemodialysis
Amoxicillin can be removed from the blood during hemodialysis.
| Hemodialysis | |
| Adults and children ≥ 40 kg | 15 mg/kg/day once. Before hemodialysis, one additional dose of 15 mg/kg is required. To restore the level of circulation of the drug after hemodialysis, it is necessary to administer an additional dose of 15 mg/kg. |
Patients receiving peritoneal dialysis
The maximum dose of amoxicillin is 500 mg per day.
Patients with liver dysfunction
Caution should be exercised and liver function should be monitored regularly.
Pharmacodynamics
Mechanism of action
Amoxicillin is a semisynthetic penicillin (beta-lactam antibiotic) that inhibits one or more enzymes (known as penicillin binding proteins - PBPs) that play a role in peptidoglycan biosynthesis. Peptidoglycan is a structural element of the bacterial cell wall. Inhibition of peptidoglycan synthesis leads to weakening of the cell wall, which is usually followed by lysis and death of the bacterial cell.
Amoxicillin is broken down by beta-lactamases, which can be produced by some bacteria, making them resistant to amoxicillin. Thus, the spectrum of activity of unprotected amoxicillin does not cover microorganisms that produce these enzymes.
Pharmacokinetics/pharmacodynamics
The time above the minimum inhibitory concentration (T > MIC) is considered the main determinant of the effectiveness of amoxicillin.
Mechanisms of resistance
The main mechanisms of resistance to amoxicillin are:
- enzymatic inactivation by beta-lactamases;
- mutation of PBP, resulting in a decrease in the affinity of the antibiotic to the target.
Impermeability of the bacterial cell wall or active removal of antibiotic from the cell (efflux) can cause or contribute to bacterial resistance in Gram-negative bacteria.
The prevalence of resistance may vary geographically and over time for certain species. It is advisable to rely on local resistance information, especially when treating severe infections. If necessary, qualified advice should be sought if the local prevalence of resistance is such that the effectiveness of the medicinal product in treating specific types of infections is questionable.
MPC boundary values
Minimum inhibitory concentration (MIC) breakpoints for amoxicillin according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) criteria, version 5.0:
| Microorganisms | MIC boundary value (mg/l) | |
| Sensitive ≤ | Resistant > | |
| Enterobacteriaceae | 81 | 8 |
| Staphylococcus spp. | note2 | note2 |
| Enterococcus spp.3 | 4 | 8 |
| Streptococci groups A, B, C and G | note4 | note4 |
| Streptococcus pneumoniae | Note5 | Note5 |
| Viridans group streptococci | 0,5 | 2 |
| Haemophilus influenzae | 26 | 26 |
| Moraxella catarrhalis | note7 | note7 |
| Neisseria meningitidis | 0,125 | 1 |
| Gram-positive anaerobes excluding Clostridium difficile8 | 4 | 8 |
| Gram-negative anaerobes8 | 0,5 | 2 |
| Helicobacter pylori | 0,1259 | 0,1259 |
| Pasteurella multocida | 1 | 1 |
| Non-species-specific breakpoints10 | 2 | 8 |
1 Wild-type Enterobacteriaceae strains are classified as susceptible to aminopenicillins. In some countries, wild-type E. coli and P. mirabilis isolates are considered moderately resistant. In this case, the MIC boundary value S ≤ 0.5 mg/l is used.
2 Most staphylococci produce penicillinases and are resistant to amoxicillin. Methidylline-resistant strains of staphylococci, with rare exceptions, are resistant to all beta-lactam antibiotics.
3 Sensitivity to amoxicillin is assessed using ampicillin.
4 The sensitivity of group A, B, C and G streptococci to penicillins is assessed based on their sensitivity to benzylpenicillin.
5 Borderline values apply to isolates isolated from all types of infection, except meningitis. If the isolate is assessed as moderately resistant to ampicillin, oral amoxicillin should not be given. Sensitivity is assessed by ampicillin MIC.
6 Breakpoints are based on intravenous administration. Strains that produce beta-lactamases are classified as resistant.
7 Microorganisms that produce beta-lactamases are classified as resistant.
8 Sensitivity to amoxicillin is assessed by sensitivity to benzylpenicillin.
9 Breakpoints are set at the epidemiological cutoff point (ECOFF) value that distinguishes wild-type isolates from those with reduced susceptibility.
10 Non-species-specific breakpoints are based on doses of 0.5 g 3 or 4 times daily (1.5 to 2 g/day).
Sensitivity of microorganisms to amoxicillin in vitro
Usually susceptible microorganisms
- Gram-positive aerobes: Enterococcus faecalis;
- Beta-hemolytic streptococci (groups A, B, C and G);
- Listeria monocytogenes.
Microorganisms that may have acquired resistance mechanisms to amoxicillin
Gram-negative aerobes: Escherichia coli; Haemophilus influenzae; Helicobacter pylori; Proteus mirabilis; Salmonella typhi; Salmonella paratyphi; Pasteurella multocida.
Gram-positive aerobes: Coagulase-negative staphylococci; Staphylococcus aureus£; Streptococcus pneumoniae; Streptococcus viridans group.
Gram-positive anaerobes: Clostridium spp.
Gram-negative anaerobes: Fusobacterium spp.
Others: Borrelia burgdorferi.
Microorganisms with Natural Resistance†
Gram-positive aerobes: Enterococcus faecium†.
Gram-negative aerobes: Acinetobacter spp.; Enterobacter spp.; Klebsiella spp.; Pseudomonas spp.
Gram-negative anaerobes: Bacteroides spp. (many strains of Bacteroides fragilis are resistant).
Others: Chlamydia spp.; Mycoplasma spp.; Legionella spp.
† Natural intermediate sensitivity in the absence of acquired resistance mechanisms.
£ Almost all strains of S. aureus are resistant to amoxicillin due to the production of penicillinases. Methicillin-resistant strains of Staphylococcus aureus (MRSA) are also resistant to amoxicillin.
Pharmacokinetics
Suction
Amoxicillin completely dissociates in aqueous solution at physiological pH. Amoxicillin is quickly and well absorbed after oral administration. When taken orally, the absolute bioavailability of amoxicillin in film-coated tablets and capsules is approximately 70%, and the time to reach maximum plasma concentration (Tmax) is 1-2 hours.
Below are the results of pharmacokinetic studies obtained when taking amoxicillin 250 mg 3 times a day in groups of healthy volunteers on an empty stomach:
| Cmax (mcg/ml) | Tmax* (h) | AUC(0-24h) (µg X h/ml) | T1/2 (h) |
| 3,3±1,12 | 1,5 (1,0-2,0) | 26,7±4,56 | 1,36±0,56 |
| * Median (range) value | |||
According to the results of comparative pharmacokinetic studies of amoxicillin in dispersible tablets and capsules, the relative bioavailability (characterized by AUC) of amoxicillin in dispersible tablets is 23% higher, the maximum plasma concentration (Cmax) is 33% higher, and the time to reach maximum plasma concentration is shorter (Tmax) - about 1 hour. Moreover, the pharmacokinetic profile of amoxicillin in dispersible tablets is equivalent to that of oral suspensions, the absolute bioavailability of amoxicillin in which can reach 98%.
Below are the results of pharmacokinetic studies obtained when taking amoxicillin 500 mg once in groups of healthy volunteers on an empty stomach:
| Amoxicillin | ||||
| dispersible tablets (whole milled) | dispersible tablets (dispersed before administration) | capsules | oral suspension | |
| Cmax (mcg/ml) | 9,2 | 9,2 | 6,9 | 9,5 |
| Tmax (min) | 68 | 58 | 88 | 61 |
| AUC0-24h (mg/ml X h) | 19,3 | 18,9 | 15,7 | 18,5 |
| 95% CI AUC0-24h | 11,3-27,3 | 12,6-25,2 | 6,6-24,7 | 6,9-30,0 |
| T1/2 (min) | 54 | 53 | 62 | 52 |
In the dose range of 250-3000 mg, bioavailability varies linearly with dose (measured by Cmax and AUC). Concomitant food intake does not affect the absorption of amoxicillin.
Hemodialysis can be used to remove amoxicillin from the circulation.
Distribution
About 18% of the total amount of amoxicillin found in plasma is bound to plasma proteins. The apparent volume of distribution is approximately 0.3-0.4 l/kg.
After intravenous administration, amoxicillin is found in the gallbladder, abdominal tissue, skin, adipose tissue, muscle, synovial and peritoneal fluids, bile and pus. Amoxicillin penetrates poorly into the cerebrospinal fluid.
Amoxicillin, like most penicillins, can be found in breast milk. Amoxicillin penetrates the placental barrier.
Biotransformation
Amoxicillin is partially excreted in the urine as inactive penicillic acid in amounts equivalent to 10-25% of the dose taken.
Removal
Amoxicillin is primarily excreted through the kidneys.
The half-life averages 1 hour, and the average total clearance is approximately 25 L/h in healthy subjects participating in the study.
Approximately 60-70% of amoxicillin is excreted unchanged in the urine during the first 6 hours after taking a single dose of 250 mg or 500 mg. In many studies, the period of excretion of 50-85% of amoxicillin in urine was 24 hours.
Concomitant use of probenecid slows down the elimination of amoxicillin.
Age
The half-life of amoxicillin is approximately the same in children aged 3 months to 2 years, in older children, and in adults.
In very young children (including premature newborns), amoxicillin is administered no more than twice a day during the first week of life, taking into account the immaturity of the renal excretion route.
Since decreased renal function may occur in the elderly, the dose should be adjusted with caution and renal function periodically monitored in this category of patients.
Floor
After oral administration of amoxicillin to males and females participating in the study, there was no significant effect of gender on the pharmacokinetics of amoxicillin.
Renal dysfunction
The total plasma clearance of amoxicillin decreases in proportion to the deterioration of renal function.
Liver failure
Caution must be exercised in patients with liver failure, and periodic monitoring of liver function is also necessary.
Indications for use Flemoxin solutab 1000 mg 20 pcs. dispersible tablets
Infections caused by microorganisms sensitive to the drug, including:
- acute bacterial sinusitis; acute otitis media;
- acute streptococcal tonsillitis and pharyngitis;
- exacerbation of chronic bronchitis;
- community-acquired pneumonia;
- acute cystitis;
- asymptomatic bacteriuria during pregnancy;
- acute pyelonephritis;
- typhus and paratyphoid;
- dental abscess with inflammation of the subcutaneous tissue;
- infections of prosthetic joints;
- Lyme disease;
- prevention of bacterial endocarditis during surgical procedures in the oral cavity and upper respiratory tract/
In combination with other drugs, according to eradication schemes, it is used to treat diseases of the digestive tract associated with Helicobacter pylori.
When choosing an antibiotic, official clinical guidelines for antibiotic therapy should be taken into account.
Contraindications
Hypersensitivity to amoxicillin, other penicillins or any other component of the drug.
History of severe immediate hypersensitivity reactions (eg, anaphylaxis) to another beta-lactam antibiotic (eg, cephalosporin, carbapenem, or monobactam).
With caution: history of allergic reactions (including bronchial asthma, polyposis, hypersensitivity to acetylsalicylic acid), history of gastrointestinal diseases (especially colitis associated with the use of antibiotics), renal failure, infectious mononucleosis, lymphocytic leukemia, pregnancy , breastfeeding period, prematurity, old age.
Application of Flemoxin solutab 1000 mg 20 pcs. dispersible tablets during pregnancy and breastfeeding
Pregnancy
Animal studies have shown no direct or indirect harmful effects in terms of reproductive toxicity. Limited data on the use of amoxicillin during pregnancy in humans does not indicate an increased risk of congenital malformations. Amoxicillin may be used during pregnancy if the potential benefit to the mother outweighs the potential risk to the fetus.
Breastfeeding period
Amoxicillin is excreted into breast milk in small quantities; if necessary, the drug can be used during breastfeeding. A breastfed baby may develop diarrhea, sensitization and fungal infection of the mucous membranes, so it may be necessary to stop breastfeeding. Amoxicillin should be used during breastfeeding only after the attending physician has assessed the benefit/risk ratio.
special instructions
Hypersensitivity reactions
Before starting treatment with amoxicillin, you should pay attention to the presence of hypersensitivity reactions to penicillins, cephalosporins or other beta-lactam antibiotics in the anamnesis (see sections “Contraindications” and “Side effects”).
Severe and sometimes fatal hypersensitivity reactions (including anaphylactic reactions and severe skin reactions) have been reported in patients receiving penicillin therapy. The development of these reactions is more likely in people with a history of hypersensitivity to penicillins and in people with atopy. If an allergic reaction occurs, discontinue treatment with amoxicillin and institute appropriate alternative treatment.
Acute coronary syndrome associated with hypersensitivity (Kounis syndrome)
In rare cases, hypersensitivity reactions (acute coronary syndrome associated with hypersensitivity) have been reported during treatment with amoxicillin. If this reaction occurs, amoxicillin should be discontinued and appropriate treatment should be prescribed.
Insensitive microorganisms
For some types of infections, before prescribing amoxicillin, it is necessary to first establish the pathogen and its sensitivity to the drug, or make sure that the pathogen is likely to be treatable with amoxicillin. This particularly applies to patients with urinary tract infections and severe ear, nose and throat infections.
Convulsions
Convulsions may occur in patients with renal failure, in patients receiving high doses of the drug, as well as in patients with predisposing factors - a history of seizures, treatment for epilepsy or meningitis, etc. (see section “Side Effects”).
Kidney failure
In patients with renal insufficiency, the dose should be adjusted according to the degree of renal insufficiency (see section "Dosage and Administration").
Skin reactions
The occurrence of generalized erythema with fever, accompanied by pustules, at the initial stage of treatment may be a symptom of OHEP (see section “Side effects”). In this case, amoxicillin should be discontinued, and its subsequent use will be contraindicated in any situation.
The use of amoxicillin should be avoided in patients who are suspected of having infectious mononucleosis, since a measles-like rash (exanthema) may occur due to the use of amoxicillin for this disease.
Jarisch-Herxheimer reaction
The Jarisch-Herxheimer reaction has been observed following the use of amoxicillin in patients with Lyme disease. This reaction is associated with the bactericidal effect of amoxicillin on the causative agent of Lyme disease, the spirochete Borrelia burgdorferi. Patients should be advised that this reaction is a common side effect of antibiotic treatment for Lyme disease and usually goes away on its own.
Excessive growth of non-susceptible microorganisms
Long-term use of the drug can sometimes lead to excessive growth of microorganisms that are not sensitive to amoxicillin (superinfection).
When using almost all antibacterial drugs, the development of colitis associated with taking antibiotics is possible. Its severity can range from mild to severe (life-threatening). Therefore, it is important to consider the possibility of this diagnosis in patients who develop diarrhea during or after antibiotic use. If diarrhea develops, the patient should immediately stop taking amoxicillin, consult a doctor and begin appropriate treatment. Medicines that inhibit peristalsis are contraindicated in this situation.
Long-term treatment
During long-term therapy, it is necessary to periodically monitor the function of the hematopoietic organs, kidneys and liver. Increased activity of liver enzymes and changes in the number of blood cells were reported.
Anticoagulants
Rare cases of increased prothrombin time have been reported in patients receiving amoxicillin. When prescribing the drug simultaneously with anticoagulants, appropriate monitoring should be carried out, and the dose of oral anticoagulants may need to be adjusted to maintain the required level of blood clotting (see sections “Interaction with other drugs” and “Side effects”).
Crystalluria
In patients with reduced diuresis, crystalluria was very rarely observed, mainly during parenteral therapy. When using high doses of amoxicillin, it is recommended to maintain adequate fluid intake and diuresis to reduce the likelihood of developing crystalluria associated with amoxicillin use. In patients with a catheterized bladder, catheter patency should be checked regularly.
Impact on diagnostic tests
Increased levels of amoxicillin in serum and urine may interfere with some laboratory tests. Due to high concentrations of amoxicillin in urine, chemical methods often give false-positive results.
When determining glucose in urine during treatment with amoxicillin, it is recommended to use enzymatic glucose oxidase tests.
The use of amoxicillin may affect the results of the quantitative determination of estradiol in urine in pregnant women.
Impact on the ability to drive vehicles and operate machinery
Studies of the effect of amoxicillin on the ability to drive vehicles or operate other machinery have not been conducted. However, side effects may occur (for example, allergic reactions, dizziness, convulsions) that affect the ability to drive vehicles or use other machinery.
Overdose
Symptoms: dysfunction of the gastrointestinal tract - nausea, vomiting, diarrhea; Vomiting and diarrhea may result in water and electrolyte imbalance.
Amoxicillin-associated crystalluria has been observed, which in some cases can lead to renal failure. Convulsions may occur in patients with impaired renal function or in patients receiving high doses of the drug.
Treatment: induce vomiting or perform gastric lavage followed by ingestion of activated charcoal and osmotic laxatives (sodium sulfate); measures are taken to restore water and electrolyte balance, hemodialysis.
Side effects Flemoxin solutab 1000 mg 20 pcs. dispersible tablets
The most common side effects are diarrhea, nausea and skin rash.
The frequency of side effects is determined as follows: very often (≥ 1/10), often (≥ 1/100,
| Infectious and parasitic diseases | |
| very rarely | candidiasis of the skin and mucous membranes |
| Blood and lymphatic system disorders | |
| very rarely | reversible leukopenia (including severe neutropenia or agranulocytosis), reversible thrombocytonenia, hemolytic anemia; prolongation of bleeding time and prothrombin time |
| Immune system disorders | |
| very rarely | severe allergic reactions such as angioedema, anaphylaxis, serum sickness and allergic vasculitis |
| unknown | Jarisch-Herxheimer reaction, acute coronary syndrome associated with hypersensitivity (Kounis syndrome) |
| Nervous system disorders | |
| very rarely | hyperkinesia, dizziness, convulsions |
| unknown | aseptic meningitis |
| Gastrointestinal disorders | |
| Clinical trial data | |
| *often | diarrhea and nausea |
| *rarely | vomit |
| Post-registration data | |
| very rarely | antibiotic-associated colitis (including pseudomembranous colitis and hemorrhagic colitis), black “hairy” tongue; superficial discoloration of teeth** |
| Disorders of the liver and biliary tract | |
| very rarely | hepatitis, cholestatic jaundice, moderate increase in the activity of aspartate aminotransferase and/or alanine aminotransferase in blood plasma |
| Skin and subcutaneous tissue disorders | |
| Clinical trial data | |
| *often | skin rash |
| *rarely | hives, itching |
| Post-registration data | |
| very rarely | skin reactions such as erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis, bullous and exfoliative dermatitis, acute generalized exanthematous pustulosis (AGEP), drug rash with eosinophilia and systemic symptoms (DRESS syndrome). |
| Renal and urinary system disorders | |
| very rarely | interstitial nephritis, crystalluria |
| * the frequency of these adverse reactions was obtained from clinical studies that included a total of about 6,000 adults and children taking amoxicillin. **Superficial tooth discoloration has been reported in children. Good oral hygiene helps prevent tooth discoloration, which can be corrected by brushing. | |
Drug interactions
Probenecid
The simultaneous use of amoxicillin and probenecid is not recommended. Probenecid reduces the secretion of amoxicillin in the renal tubules. Concomitant use of probenecid may lead to increased concentrations of amoxicillin in the blood.
Allopurinol
Concomitant use of allopurinol during treatment with amoxicillin increases the likelihood of developing allergic skin reactions.
Tetracyclines
Tetracyclines and other bacteriostatic antibiotics may interfere with the bactericidal effect of amoxicillin.
Oral anticoagulants
Oral anticoagulants and penicillin-based antibiotics are often used together in practice, with no reports of interaction. However, the literature describes cases of increased international normalized ratio in patients receiving treatment with acenocoumarol or warfarin during a prescribed course of amoxicillin.
If simultaneous administration of drugs is necessary, it is necessary to carefully monitor the prothrombin time or international normalized ratio at the beginning of treatment and after discontinuation of treatment with amoxicillin. In addition, dosage adjustments of oral anticoagulants may be necessary.
Methotrexate
Penicillin antibiotics may reduce the excretion of methotrexate, which may be accompanied by increased toxicity.
Side effects
From the gastrointestinal tract: rarely - changes in taste, nausea, vomiting, diarrhea; in some cases - a moderate increase in the activity of liver transaminases; extremely rarely - pseudomembranous and hemorrhagic colitis.
From the urinary system: extremely rarely - the development of interstitial nephritis.
From the hematopoietic system: agranulocytosis, neutropenia, thrombocytopenia, hemolytic anemia are possible, but they are also extremely rare.
Side effects from the nervous system when using amoxicillin in the dosage form of dispersible tablets have not been registered.
Allergic reactions: skin reactions, mainly in the form of a specific maculopapular rash; rarely - exudative erythema multiforme (Stevens-Johnson syndrome); in some cases - anaphylactic shock, angioedema.
Flemoxin Solutab tab 125 mg N20 (Astellas)
Hypersensitivity reactions
Before starting treatment with amoxicillin, you should pay attention to the presence of hypersensitivity reactions to penicillins, cephalosporins or other beta-lactam antibiotics in the anamnesis (see sections “Contraindications” and “Side effects”).
Severe and sometimes fatal hypersensitivity reactions (including anaphylactic reactions and severe skin reactions) have been reported in patients receiving penicillin therapy. The development of these reactions is more likely in people with a history of hypersensitivity to penicillins and in people with atopy. If an allergic reaction occurs, discontinue treatment with amoxicillin and institute appropriate alternative treatment.
Acute coronary syndrome associated with hypersensitivity (Kounis syndrome)
In rare cases, hypersensitivity reactions (acute coronary syndrome associated with hypersensitivity) have been reported during treatment with amoxicillin. If this reaction occurs, amoxicillin should be discontinued and appropriate treatment should be prescribed.
Insensitive microorganisms
For some types of infections, before prescribing amoxicillin, it is necessary to first establish the pathogen and its sensitivity to the drug, or make sure that the pathogen is likely to be treatable with amoxicillin. This particularly applies to patients with urinary tract infections and severe ear, nose and throat infections.
Convulsions
Convulsions may occur in patients with renal failure, in patients receiving high doses of the drug, as well as in patients with predisposing factors - a history of seizures, treatment for epilepsy or meningitis, etc. (see section “Side Effects”).
Kidney failure
In patients with renal insufficiency, the dose should be adjusted according to the degree of renal insufficiency (see section "Dosage and Administration").
Skin reactions
The occurrence of generalized erythema with fever, accompanied by pustules, at the initial stage of treatment may be a symptom of OHEP (see section “Side effects”). In this case, amoxicillin should be discontinued, and its subsequent use will be contraindicated in any situation.
The use of amoxicillin should be avoided in patients who are suspected of having infectious mononucleosis, since a measles-like rash (exanthema) may occur due to the use of amoxicillin for this disease.
Jarisch-Herxheimer reaction
The Jarisch-Herxheimer reaction has been observed following the use of amoxicillin in patients with Lyme disease. This reaction is associated with the bactericidal effect of amoxicillin on the causative agent of Lyme disease, the spirochete Borrelia burgdorferi. Patients should be advised that this reaction is a common side effect of antibiotic treatment for Lyme disease and usually goes away on its own.
Excessive growth of non-susceptible microorganisms
Long-term use of the drug can sometimes lead to excessive growth of microorganisms that are not sensitive to amoxicillin (superinfection).
When using almost all antibacterial drugs, the development of colitis associated with taking antibiotics is possible. Its severity can range from mild to severe (life-threatening). Therefore, it is important to consider the possibility of this diagnosis in patients who develop diarrhea during or after antibiotic use. If diarrhea develops, the patient should immediately stop taking amoxicillin, consult a doctor and begin appropriate treatment. Medicines that inhibit peristalsis are contraindicated in this situation.
Long-term treatment
During long-term therapy, it is necessary to periodically monitor the function of the hematopoietic organs, kidneys and liver. Increased activity of liver enzymes and changes in the number of blood cells were reported.
Anticoagulants
Rare cases of increased prothrombin time have been reported in patients receiving amoxicillin. When prescribing the drug simultaneously with anticoagulants, appropriate monitoring should be carried out, and the dose of oral anticoagulants may need to be adjusted to maintain the required level of blood clotting (see sections “Interaction with other drugs” and “Side effects”).
Crystalluria
In patients with reduced diuresis, crystalluria was very rarely observed, mainly during parenteral therapy. When using high doses of amoxicillin, it is recommended to maintain adequate fluid intake and diuresis to reduce the likelihood of developing crystalluria associated with amoxicillin use. In patients with a catheterized bladder, catheter patency should be checked regularly.
Impact on diagnostic tests
Increased levels of amoxicillin in serum and urine may interfere with some laboratory tests. Due to high concentrations of amoxicillin in urine, chemical methods often give false-positive results.
When determining glucose in urine during treatment with amoxicillin, it is recommended to use enzymatic glucose oxidase tests.
The use of amoxicillin may affect the results of the quantitative determination of estradiol in urine in pregnant women.
Interaction
Probenecid, phenylbutazone, oxyphenbutazone, and to a lesser extent acetylsalicylic acid and sulfinpyrazone, inhibit the tubular secretion of penicillins, which leads to an increase in the half-life and an increase in the concentration of amoxicillin in the blood plasma. Bactericidal antibiotics (including aminoglycosides, cephalosporins, vancomycin, rifampicin) have a synergistic effect when taken simultaneously; antagonism is possible when taken with some bacteriostatic drugs (for example, chloramphenicol, sulfonamides). Concomitant use with estrogen-containing oral contraceptives may lead to a decrease in their effectiveness and an increased risk of breakthrough bleeding). Concomitant administration with allopurinol does not increase the frequency of skin reactions, unlike the combination of allopurinol with ampicillin.
Directions for use and doses
Inside, before, during or after meals. The tablet can be swallowed whole, divided into pieces or chewed with a glass of water or diluted in water to form a syrup (20 ml) or suspension (100 ml).
Adults and children over 10 years of age (for mild to moderate infections) - 500–750 mg 2 times a day or 375–500 mg 3 times a day.
Children from 3 to 10 years old - 375 mg 2 times a day or 250 mg 3 times a day; from 1 year to 3 years - 250 mg 2 times a day or 125 mg 3 times a day. The daily dose for children (including children under 1 year of age) is 30–60 mg/kg/day, divided into 2–3 doses.
When treating severe infections, as well as for infections with hard-to-reach foci (for example, acute otitis media), a three-time dose of the drug is preferable.
For chronic diseases, relapses, severe infections: adults - 0.75–1 g 3 times a day, children - up to 60 mg/kg/day in 3 divided doses.
For acute uncomplicated gonorrhea - 3 g, once, in combination with 1 g probenecid.
For mild to moderate infections, treatment is carried out for 5-7 days, for infections caused by Streptococcus pyogenes - at least 10 days.
When treating chronic diseases and severe infections, the dose of the drug should be determined by the clinical picture of the disease. The drug is continued for 48 hours after the symptoms of the disease disappear.
For patients with creatinine Cl below 10 ml/min, the dose is reduced by 15–50%.
Acute streptococcal infection of the oropharynx in pediatric practice - problem and solutions
The extreme prevalence of group A streptococcus (GAS) as a respiratory pathogen, its many serotypes, strictly type-specific formation of post-infectious immunity and ease of transmission determine the total prevalence of streptococcal infections in children, especially in organized groups [1]. There are standards for the treatment of scarlet fever, as well as tonsillitis (the latter is streptococcal in nature in 70% of cases according to the Novosibirsk Infectious Diseases Hospital) based on the use of etiotropic, pathogenetic and symptomatic agents. Numerous studies have shown that GAS has remained sensitive to penicillin drugs for more than 50 years due to the fact that it does not secrete penicillinase, like other pathogens. However, in cases of mixed infection, penicillins are ineffective; with irrational therapy or in cases of frequent reinfection with new serotypes of GAS with genotypically determined characteristics of the reactivity of the child’s body (sensitization with the development of immunopathological reactions), complications may develop in the form of rheumatism, glomerulonephritis and other immunoinflammatory processes.
Infectious and immune-mediated diseases associated with streptococcus:
- superficial forms - sore throat, pharyngitis, streptoderma, erysipelas;
- deep forms (invasive) - phlegmon, myositis, pericarditis, endocarditis, meningitis, pneumonia, peritonitis, sepsis;
- toxin-mediated forms - scarlet fever, toxic shock syndrome;
- immunopathological forms - rheumatism, arthritis, post-streptococcal glomerulonephritis, vasculitis.
Based on the nature of hemolysis in blood agar, streptococci are classified into alpha, beta and gamma. Alpha (green) and gamma streptococci do not lyse red blood cells and are called non-hemolytic, i.e. they are non-pathogenic for humans. They are widely represented in the normal microflora of the oral cavity (oral) and large intestine (enterococci). Beta-hemolytic streptococci are classified as pyogenic, i.e. they are pathogenic. Rarely isolated from healthy ones and are a potential threat to the owner.
Human immunity to streptococcal infections is due to antibodies to the M-antigen. There are more than 80 serotypes of GAS based on the M protein, and antibacterial immunity is narrowly type-specific. Each M-serotype produces its own agglutinins, precipitins, and complement-fixing antibodies, which is why reinfections are possible, i.e., repeated diseases as a result of infection with new serotypes.
The number of hospitalized children with tonsillitis according to the children's city clinical hospital No. 3 of Novosibirsk in 2008 was 740, in 2009 - 1190, in 2010 - 1438 people, i.e. 11% of all nosological forms. Both boys and girls are equally affected. The distribution of patients by age turned out to be interesting: fewer children were admitted aged 7–10 years and older (perhaps they were treated at the site). It is interesting that the proportion of children in the first three years of life has increased over the past three years (given the imperfection of the lymphoid-pharyngeal ring in this age category). Until now, sore throat was an uncharacteristic, almost casuistic phenomenon, especially in children in the first year of life. In 2010, patients under three years of age accounted for 45% of hospitalized patients, including 9% of children under one year of age.
Traditionally, bacteriological diagnosis of Streptococcus pyogenes (routine method) includes:
- Culture from the oropharynx on a plate with blood agar (BA) - 5% defibrinated sheep blood.
- Sowing technique. The material is applied to 1/6 of the surface of the spacecraft, then using a loop, seeding is done in streaks in four quadrants.
- Incubation is possible under normal atmosphere conditions, but it is better to incubate crops with 5–7% CO2 content. The optimal incubation temperature is 35–37 °C.
- Morphological characteristics - the presence of beta-hemolysis, the diameter of the colonies is 1–2 mm.
- Phenotypic methods - catalase reaction.
- Sensitivity to 0.04 units of bacitracin.
- PYR test.
In 2009, we conducted a retrospective analysis of 300 case histories of children diagnosed with lacunar tonsillitis. The culture rate of S. pyogenes was approximately 20%; other beta-hemolytic streptococci could not be identified to species using routine techniques, and their diagnostic value generally remains unclear. The American Academy of Pediatrics for the detection of S. pyogenes in children with acute tonsillopharigitis, due to the insufficient information content of the routine bacteriological diagnostic method, recommends a rapid diagnostic method with duplicate microbiological examination of the material using the OSOM Ultra Strep A Test or the method of two sequential rapid tests [2].
In Russia, express methods are not available to every laboratory, therefore, to improve the diagnosis of S. pyogenes and other beta-hemolytic streptococci in the hospital, we conduct a double sequential microbiological study. We use Brain Heart infusion Broth HiMedia (brain heart broth) as an enrichment system, which is usually used to cultivate “fastidious” microorganisms. To identify most streptococci to species, the STREPTOtest 16 Pliva-Lachema microtest systems and the VAST v 3.5 computer program are designed.
Primary seeding of S. pyogenes was 60 of 254–20.4%. When reseeding through enrichment medium, an additional 36 strains were obtained, which amounted to another 14.2%. Thus, a total of 96 S. pyogenes strains were obtained and the overall inoculation rate was 37.4% (Table 1).
Thanks to the STREPTOtest 16 test systems, it was possible to identify additional representatives of beta-hemolyzing streptococci, and with the help of additional subculture and the use of the BACT program, we also obtained representatives of the genera Moraxella spp., Haemophilus spp., S. pneumoniae [3]. The possibility of increasing the efficiency of microbiological diagnosis of streptococcal infection of the oropharynx in children lies in the use of the method of repeated seeding on enrichment media. Thus, hemolytic streptococcus is much more often the etiological agent of sore throat in children than is confirmed by routine bacteriological methods (in every third hospitalized patient).
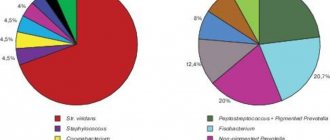
At the next stage of the study, out of 254 children, we selected 96 with S. pyogenes seeding (Table 2). In 75% of children with streptococcal tonsillitis, pyogenic streptococcus was combined with pathogens that secrete beta-lactamases (Staphylococcus aureus, pneumococcus, Haemophilus influenzae, Moraxella, Pseudomonas aeruginosa, opportunistic enterobacteria, coagulase-negative staphylococcus, candida), which may contribute to the failure of penicillin therapy.
Antibacterial therapy
The goal of antibacterial therapy for acute streptococcal tonsillitis is eradication of the pathogen, which leads not only to the elimination of the symptoms of the infection, but also to the prevention of its spread, and prevents early and late complications. Peritonsillar abscess and purulent cervical lymphadenitis in the pre-penicillin era developed in 13% of hospitalized patients, but are now rare. The probability of developing rheumatism was 2.1% in the 40s, and with the advent of antibacterial treatment - 0.3% [1]. Prescribing antibiotics prevents the spread of streptococcal infection, reducing the number of carriers of the pathogen.
Penicillins, aminopenicillins, cephalosporins are indicated. In patients with a proven allergy to beta-lactams, macrolides should be used, and in case of intolerance to the latter, lincosamides. GAS are highly sensitive to penicillins and cephalosporins.
The route of administration for systemic antibiotic therapy should provide the necessary concentration of the drug at the site of infection, be simple and not burdensome for the child. For outpatients, antibiotics are usually prescribed orally, except in cases where one intramuscular injection is sufficient. In the hospital, the antibiotic is often administered intramuscularly (in the absence of blood clotting disorders), and in severe forms and the possibility of venous catheterization - intravenously. Parenteral administration of antibiotics should be used at the beginning of treatment, and as soon as the patient’s condition improves, switch to taking the drug orally. In pediatrics, this provision is especially important for reducing negative reactions on the part of the child.
Penicillins are the first-line drugs in the treatment of infectious processes caused by streptococcus pyogenes, both in Russia and abroad. Due to the fact that SGA is the most likely etiological agent for angina, it is necessary to start therapy with one of these drugs (empirically), and after receiving the results of bacterial cultures from the throat, further adjustments must be made. Penicillins are used in a dose of 100–150 thousand units/kg/day. No data have been obtained on the resistance of GAS to penicillins. The basis of action of penicillins and beta-lactams is inhibition of cell wall synthesis and a bactericidal effect. Benzylpenicillin is used parenterally 6 times a day, which cannot be provided in an outpatient setting. Phenoxymethylpenicillin (penicillin V) is administered enterally one hour before meals or 2 hours after meals (when interacting with food, a decrease in bactericidal concentration in plasma is observed) 0.375 g in 2 doses (< 25 kg), 750 mg in 2 doses (> 25 kg ).
The level of amoxicillin in the tonsils is 3 times higher than the level of equal doses of phenoxymethylpenicillin and ampicillin. It has a longer half-life, so it is prescribed 2-3 times a day. Food does not affect the bioavailability of the drug. The dosage form of amoxicillin Flemoxin Solutab penetrates well into the tonsil tissue, is prescribed 0.375 g in 2 doses (< 25 kg), 750 mg in 2 doses (> 25 kg).
Amoxicillin-clavunate - the presence of the beta-lactamase inhibitor clavulanic acid prevents the enzymatic degradation of amoxicillin, increases the activity of the drug against gram-positive and gram-negative aerobic and anaerobic microorganisms that produce these enzymes. Amoxiclav (Lek, Slovenia), Augmentin (SmithKline Beecham, UK).
When using aminopenicillins in treatment, it must be remembered that in pediatric practice there is a contraindication to the prescription - infectious mononucleosis. There is a high risk of developing a rash (90–100%) of immunocomplex origin due to the formation of immune complexes from the amino group of the antibiotic (hapten), IgM to the Epstein–Barr virus and immune complexes. Sore throat is one of the first symptoms of infectious mononucleosis, and therefore is often the initial diagnosis. When treated with aminopenicillin, not immediately, but after a few days (when antibodies to the virus begin to appear), a generalized maculopapular rash occurs, and the patient’s condition worsens. Therefore, early differential diagnosis of angina and infectious mononucleosis is important for the rational choice of a drug.
1st and 2nd generation cephalosporins are antibiotics containing a lactam ring. Inhibits the synthesis of cell wall peptidoglycans. The spectrum of action includes most gram-positive bacteria, including not only streptococci, but also staphylococci. With each subsequent generation, their activity against gram-negative bacteria increases, and against cocci it decreases (with the exception of ceftriaxone, which is highly active against cocci). 1st generation drugs act only on coccal flora. Currently rarely used. 2nd generation drugs have a wider spectrum: in addition to cocci, they suppress the growth of some strains of ampicillin-resistant pathogens (M. catarrhalis, H. influenzae, S. pneumoniae). Cefuroxime-axetine (2nd generation) is prescribed 30 mg/kg/day IM, IV or orally 2 times a day. Tablets are available in 125, 250 and 500 mg.
Usually, patients with streptococcal tonsillitis do not need to be prescribed 3rd generation cephalosporins, however, 2-3 cases of severe damage to the pharynx with extensive purulent deposits and necrosis are observed annually. At the same time, there is a low effectiveness of the use of penicillins and cephalosporins of the 1st and 2nd generation - the persistence of fever, purulent-inflammatory process at the gates of infection. Throat cultures provide little information: pyogenic streptococcus, sensitive to traditional drugs, is isolated, but no effect is observed in therapy. The reason for this, according to our data, is another bacterial infection associated with streptococcus with beta-lactamase activity (pneumococci, candida, moraxella, hemophilus, etc.) or anaerobic (bacteriodes, including peptococci, peptostreptococci, fusobacteria, etc.) and a decrease "mucosal" immunity.
3rd generation drugs - cefotaxime, ceftazidime and ceftriaxone - have pronounced activity against M. catarrhalis, H. influenzae, including strains with reduced sensitivity, regardless of the type of lactamase.
Ceftazidime (Fortum), often in combination with aminoglycosides, is the first choice drug for infections caused by Pseudomonas aeruginosa. Prescribed IV, IM 100–150 mg/kg/day once. In 19% of children with tonsillitis syndrome, in addition to hemolytic streptococcus, Pseudomonas aeruginosa was isolated from the throat (Table 2). Of great interest is the fact that these children were hospitalized not from other hospitals, where Pseudomonas aeruginosa infection usually spreads, but from their place of residence, where they had contact with long-term ill relatives (grandparents who received courses of antibiotic treatment), who also discharged from the throat this pathogen.
Ceftriaxone has a half-life of 7 hours and can be administered once a day IV, IM 20–80 mg/kg/day.
Cefotaxime - IV, IM 50–100 mg/kg/day for infections caused by any type of lactamase, as well as in patients who have previously received antibiotics.
Cefixime (Suprax) is a drug for oral administration in the form of capsules or suspension, convenient for use in pediatric practice, including for sore throat in cases of the presence of several infectious agents in the oropharynx. For children under 12 years of age, the drug is prescribed in suspension at a dose of 8 mg/kg once a day or 4 mg/kg every 12 hours. For children from 6 months to one year, the daily dose is 2.5–4 ml; from 2–4 years – 5 ml; from 6–11 years – 6–10 ml of suspension. For adults and children over 12 years of age weighing more than 50 kg, the daily dose is 400 mg once a day, or 200 mg/2 times a day. The duration of treatment is 7–10 days.
Macrolides are active against coccal flora, diphtheria pathogens, and anaerobes (except B. fragilis), but all of them, except azithromycin, are inactive against Haemophilus influenzae. They accumulate well in cells, where their concentration exceeds that in blood serum.
Azithromycin (Sumamed) is a type of azalide, resistant to the acidic environment of the stomach, creating high concentrations in the tonsils. A feature of pharmacokinetics is a long half-life from tissues (inhibition of cytochrome P450 in the liver). Bactericidal concentrations in the tonsils remain for another 7 days after discontinuation of the drug. It is prescribed once a day at a dose of 10 mg/kg, from the 2nd day 5 mg/kg for 5 days. Food slows down absorption (recommended to be taken an hour before meals or 2 hours after).
Josamycin, midecamycin (Macropen) - 40–50 mg/kg/day.
Clarithromycin, roxithromycin - 6-8 mg/kg/day orally.
Spiramycin (Rovamycin) - 100 units/kg 2 times a day orally.
Erythromycin - IV 20–50 mg/kg/day, orally 50 mg/kg/day, maximum 1–2 g/day.
The course of antibacterial therapy for angina syndrome with the release of beta-hemolytic streptococcus is at least 10 days. Shorter courses often lead to relapses of acute tonsillitis and re-hospitalization of patients.
Local antibacterial agents
Due to the fact that it is impossible to give a detailed review of topical drugs, we will focus on those products whose effectiveness has been confirmed by our own experience.
Local drugs for angina must necessarily be an addition to the antimicrobial therapy system, i.e. their role is secondary.
Fusafungin (Bioparox) is a local inhaled antimicrobial drug that can be prescribed from the first day of illness until the results of a microbiological study are obtained. It has a wide spectrum of antimicrobial action, its own anti-inflammatory properties, lack of absorption from the mucous membrane, low allergenicity, i.e., it meets all the requirements for local antibacterial agents. The most optimal is to use the drug from 1 to 4 doses, depending on age, every 4 hours for 10 days.
Tonsilgon N is a combined preparation of herbal origin. The chamomile, marshmallow and horsetail components included in its composition stimulate the body's defenses by increasing the phagocytic activity of macrophages and granulocytes. The drug has anti-inflammatory, immunostimulating, anti-edematous and antiviral effects, accelerates the healing process, and can later be used to prevent relapses of tonsillitis. There were no side effects when using the drug. Tonsilgon N is available in two forms: drops for oral administration and tablets. For adults, the drug is prescribed 25 drops or 2 tablets 5-6 times a day, children under 5 years old 5-10 drops, from 6-10 years old 15 drops, 11-16 years old - 20 drops 5-6 times a day. After the disappearance of acute manifestations of the disease, the frequency of taking Tonsilgon N is reduced to 3 times a day. The duration of basic therapy for PBD for recurrent tonsillitis and chronic tonsillitis can last 4–6 weeks.
Hexetidine (Hexoral) is available both as a rinse solution and as an aerosol. Unlike chlorhexidine, the drug is low-toxic. Active against most bacteria - the causative agents of tonsillopharyngitis, as well as fungi. In addition to antimicrobial, it has hemostatic, analgesic and deodorizing effects.
Octenisept is an antiseptic for mucous membranes with the widest spectrum of antimicrobial action, covering gram-positive and gram-negative bacteria, chlamydia, mycoplasma, fungi, protozoa and even viruses of the herpes family. The effect of the drug begins within a minute and lasts for an hour. It is non-toxic and is not absorbed through intact mucous membranes. The drug can be sprayed onto the mucous membranes using an insufflator (for rinsing or spraying, dilute 1:10).
Aqua Maris is a spray for the throat and nose to cleanse, relieve irritation and protect the mucous membrane of the nasopharynx. Composition: the bottle contains 30 ml of a sterile hypertonic solution of Adriatic Sea water.
Ion content: Na+, K+, Ca2+, Cl-, Mg2+, SO42-, HCO3, Br-. Does not contain preservatives. Mechanisms of action: washing away bacteria and viruses from the tonsils and posterior wall of the nasopharynx, antiseptic effect, activation of local immunity.
Directions: adults and children 4-6 times a day, 3-4 injections, directing the sprayer to the back wall of the pharynx.
Strepsils - lozenges, contains amylmetacresol and dichlorobenzyl alcohol, which have antiseptic, anti-inflammatory and analgesic properties, as well as menthol and eucalyptus, anise oil, honey, lemon, vitamin C. Active against gram-positive and gram-negative microflora. Mode of application:
- for children over 5 years old, 1 tablet every 2–3 hours, but not more than 8 tablets in 24 hours;
- dissolve until completely dissolved;
- It is advisable not to drink or eat for a while after dissolving the tablet.
Of course, the most effective local drugs will not completely replace the need for systemic administration of antibiotics for sore throat. However, in case of undesirable effects of general antibiotic therapy, local administration of drugs with a wide spectrum of antimicrobial activity is the method of choice.
Anti-inflammatory and antipyretic drugs
Fever and pain associated with the development of inflammatory manifestations in the pharynx are the main clinical signs of tonsillitis. Fever with a temperature of less than 39°C in healthy children generally does not require treatment. However, with streptococcal sore throat, fever often manifests itself and is combined with manifestations of intoxication, which significantly worsens the well-being of patients.
Antipyretic therapy is indicated:
- Previously healthy: - at t > 39 °C; - for muscle aches; - for headaches.
- With a history of convulsions at t > 38 °C.
- For severe chronic diseases (t > 38 °C).
- In the first 3 months of life (t > 38 °C).
Prescribing acetylsalicylic acid (Aspirin) for this purpose to children and adolescents has been prohibited in the USA since the 70s, and in Russia since the late 90s, due to the proven connection of their use with the development of Reye's syndrome, which has a high mortality rate (Pharm Committee order dated March 25 .1999); Aspirin remains in practice as a drug effective for rheumatological disorders.
Analgin is not used as an over-the-counter antipyretic, which is associated with the risk of developing agranulocytosis and collapse with hypothermia; this drug is prescribed only as an analgesic or for quickly reducing temperature for special indications as part of a lytic mixture: IM Analgin 50% solution 0.1–0.2 ml/10 kg + papaverine 0.1–0.2 ml 2 % solution.
Paracetamol is a frequently used antipyretic and mild analgesic in children, a derivative of phenacetin, but much less toxic than the latter. The main mechanism of the antipyretic effect is inhibition of prostaglandin synthesis by reducing the activity of cycloxygenase in the hypothalamus. Paracetamol inhibits the “cerebral” synthesis of prostaglandins to a greater extent than the “peripheral” one, does not have an antiplatelet effect, and does not cause bleeding like Aspirin.
Paracetamol is metabolized in the liver and has low toxicity in recommended dosages. The daily total dose of paracetamol for oral or rectal use should not exceed 100 mg/kg per day in children over one year of age, 75 mg/kg in infants. It is not recommended in combination with drugs that, like paracetamol itself, under the influence of cytochrome P 450 are capable of being converted into “reactive metabolites” in the liver and kidneys and damaging the latter (rifampicin, phenobarbital, antiepileptic drugs). Contraindicated for liver diseases. Exceeding recommended doses can lead to liver failure and hepatic encephalopathy due to the formation of excess “reactive metabolite”. Acute renal failure (acute renal tubular necrosis) is also possible. Pediatricians may be surprised by how often over-the-counter antipyretics are used in practice. According to a survey of mothers in the United States from 1994 to 2000, more than half of mothers gave over-the-counter antipyretics and analgesics to infants in the last 30 days before the survey, with 2/3 of children receiving acetaminophen (paracetamol). It has been established that parents are not able to measure the exact doses of liquid medications that are most often used in the treatment of young children. They believed that antipyretics intended for children in the first three years of life are less concentrated (i.e., contain less active substance in solution) than those used in children of older age groups. In fact, they were more concentrated to make it easier to measure the small doses required for young children. The described “confusion” led to overdoses and even death of children [4]. In Russia, this problem is no less urgent, because with persistent fever and a short period of apyrexia, parents overestimate paracetamol doses for children in 40% of cases, wanting to get a faster and longer-lasting analgesic result [5]. The safety of paracetamol for children can only be ensured by strict adherence to the instructions for its use.
Ibuprofen (Nurofen for children, Nurofen) - a derivative of propionic acid - has antipyretic, analgesic and anti-inflammatory properties. Currently used in more than 30 countries. Nurofen for children (ibuprofen) is a non-steroidal anti-inflammatory drug (NSAID) and is indicated for the reduction of high fever, as well as for the relief of mild to moderate pain, such as a sore throat due to a sore throat, headache as a symptom of a sore throat. Febrile fever is one of the leading manifestations of the disease in acute GAS infection (sore throat, scarlet fever), so there is often a need to obtain an antipyretic effect. But, in addition, there are pronounced inflammatory manifestations in the pharynx: 1) bright hyperemia of the tonsils, arches, uvula, and posterior pharyngeal wall; 2) hypertrophy of the tonsils, associated mainly with their infiltration with polynuclear cells and, to a lesser extent, with edema; 3) plaque on the tonsils as an exudative component of the local inflammatory reaction; 4) pain due to inflammatory manifestations in the pharynx. In cases of severe inflammation in the oropharynx and regional lymph nodes, as well as in cases of recurrence of acute tonsillitis caused by beta-hemolytic streptococcus, the inclusion of anti-inflammatory drugs in the complex of therapy is pathogenetically justified. For this purpose, non-steroidal anti-inflammatory drugs are currently widely used in clinical practice. They have a unique combination of anti-inflammatory, analgesic and antipyretic mechanisms of action. The therapeutic effect of non-steroidal anti-inflammatory drugs is based on the mechanisms of inhibition of prostaglandin synthesis by reducing the activity of cycloxygenase (COX), an enzyme that regulates the conversion of arachidonic acid into prostaglandins.
Nurofen is available in the form of: 1) suspension (in a 100 ml bottle and a measuring syringe) with a pleasant orange or strawberry flavor, which contains 100 mg/5 ml of ibuprofen (no sugar, alcohol or artificial colors); 2) film-coated tablets (200 mg of ibuprofen in 1 tablet); 3) rectal suppositories (60 mg of ibuprofen in 1 suppository).
With the development of acute streptococcal infection, a 7-10-day course of antibacterial therapy ensures the body is sanitized from the pathogen, but the pro-inflammatory activity of the body’s endogenous intensifying system (cytokine “cascade”, synthesis of prostaglandins, leukotrienes, reactive oxygen species, etc.) can contribute to significant damage tissues at the site of inflammation and subsequent long-term persistence of the inflammatory process. The study of the anti-inflammatory effect of non-steroidal anti-inflammatory drugs in the complex of treatments for acute streptococcal infections in children is of undoubted interest. In 2010, we conducted a study to study the anti-inflammatory effect of ibuprofen in children with acute streptococcal infection of the oropharynx, depending on different treatment regimens: 10-day antibacterial with the inclusion of a course of Nurofen for children in the first 5 days of therapy (studied group of 30 people in ages from 3 to 12 years) or without it (control group of 26 people of the same age) [6].
The safety of Nurofen for children is due to:
- short half-life (1.8–2 hours);
- during metabolism in the liver, pharmacologically active substances are not formed, so there is no direct toxic effect on parenchymal organs (liver, kidneys, etc.);
- excretion of drug metabolites in urine is completed 24 hours after taking the last dose. The rapid metabolism and excretion of ibuprofen to some extent explain its relatively low toxicity compared to other NSAIDs and the lack of negative effects on renal function. With long-term use, its accumulation in the body does not occur.
In addition to antibacterial drugs, Nurofen for children was used by patients in the experimental group 3–4 times a day during the first 5 days of therapy in a standard single dose of 5–10 mg/kg, which more often ranged from 2.5 to 5 ml of suspension per dose. The antipyretic and anti-inflammatory effects of Nurofen, as well as its safety, were assessed. Children in the control group, when a fever above 38.5 °C appeared, received paracetamol in a single dose of 10–15 mg/kg, i.e., in the form of symptomatic therapy as needed. As is known, paracetamol is not a non-steroidal anti-inflammatory drug, but belongs to the group of “simple analgesics”, since it has antipyretic and analgesic effects, and its anti-inflammatory activity is insignificant. In children receiving Nurofen, stable normalization of temperature, clearing of palatine tonsils from plaque, a decrease in the degree of tonsil hypertrophy, and regression of regional lymphadenitis occurred more quickly than in the comparison group. Complications in the form of a peritonsillar abscess in one 8-year-old child with streptococcal tonsillitis and acute sinusitis in one 6-year-old child with a mixed infection were noted in the second group, while in the first group no complications were observed. Adverse events (allergic rash) were noted in one patient of the first group and one patient in the second. Thus, Nurofen appears to be a highly effective antipyretic and anti-inflammatory drug used for sore throat in children.
Nurofen for children in the form of a suspension is given to children 3–12 months old, 2.5 ml no more than 3–4 times a day (no more than 200 mg/day); 1–3 years - 5 ml 3 times a day (no more than 300 mg/day); 4–6 years - 7.5 ml 3 times a day (no more than 450 mg/day); 7–9 years - 10 ml 3 times a day (no more than 600 mg/day); 10–12 years - 15 ml 3 times a day (no more than 900 mg/day). The tablet form of Nurofen is used in children over 6 years of age weighing more than 20 kg in the same doses as syrup, but not more than 4 tablets/800 mg of ibuprofen per day. The maximum daily dose should not exceed 30 mg/kg of the child's body weight.
If you have a history of allergic diseases and concomitant pathology of the digestive organs, it is rational to use paracetamol or Nurofen in suppositories due to the absence of flavoring additives in the rectal form and a direct effect on the gastric mucosa.
Nurofen for children is available in suppositories of 60 mg/1 supp. Intended for use in children from 3 months, a single dose is 5–10 mg/kg. If the fever is not a short-term episode and persists for a day or more, Nurofen is prescribed to children from 3 to 9 months of age 1 suppository 3 times a day (no more than 180 mg per day), from 9 months to 2 years - 1 suppository 4 once a day (no more than 240 mg/day).
In children with regurgitation and vomiting syndrome, it is advisable to use ibuprofen in rectal form, which eliminates direct effects on the gastric mucosa and the possibility of drug overdose. The absence of flavoring additives in candles prevents the development of allergic reactions in children with an unfavorable allergy history.
Prevention of recurrence of streptococcal infections
In the 50s of the 20th century, due to the prevailing circulation of rheumatogenic strains of SGA, the Ministry of Health of the Russian Federation issued an order on mandatory one-time bicillin prophylaxis for all children who had streptococcal tonsillitis or scarlet fever after a 10-day course of antibiotic treatment. This order has not been canceled to this day, despite the fact that cases of rheumatism have been rarely recorded in recent years, domestic bicillins-3 and 5 are multicomponent and require improvement (their introduction leads to the formation of peak concentrations in the blood in the first days with a rapid loss of the bactericidal effect in dynamics). In the reference book on drug therapy by V.K. Tatochenko “For a pediatrician for every day” (p. 125), the Decision of the antibiotic commission of the Ministry of Health of the Russian Federation and the Russian Academy of Medical Sciences “Antibacterial therapy of streptococcal tonsillitis (acute) and pharyngitis” appeared. Guidelines. M., 1999: “Bicillins are prescribed if it is impossible to carry out a 10-day course of treatment, with a rheumatic history, as well as during outbreaks of infection caused by beta-hemolytic streptococci of group A in groups. For acute A-streptococcal tonsillitis in patients with risk factors for the development of acute rheumatic fever (complicated heredity, unfavorable social and living conditions, etc.), it is advisable to use benzylpenicillin for 10 days, followed by a single injection of benzathylbenzylpenicillin. In other cases, only a 10-day course of antibiotics is necessary.” However, the regions did not receive regulatory instructions to cancel the old order, and therefore many clinics and hospitals continue to implement it. The use of immunomodulators, including bacterial lysates, as well as agents that normalize the biocenosis of the oral cavity, is the most important way to prevent relapses of streptococcal infections of the oropharynx.
Literature
- Pokrovsky V. I., Briko N. I., Ryapis L. A. Streptococci and streptococcosis. M.: Geotar-media, 2008. 540 p.
- Gieseker KE Evaluating the American Academy Pediatrics diagnostic standard for Streptococcus pyogenes pharyngitis: Backup culture versus repeat rapid antigen testing // Pediatrics. 2003; 111:66–70.
- Krasnova E.I., Chretien S.O. Optimization of therapy for streptococcal infections of the oropharynx using bacterial lysates // Children's infections. 2011, vol. 10, no. 1, p. 52–56.
- Dlugosz CK, Chater RW, Engle JP Appropriate Use of Nonprescription Analgesics in Pediatric Patients // J Pediatr Health Care. 2006; 20 (5): 316–325.
- Geppe N. A., Zaitseva O. V. Understanding the mechanisms of fever in children and the principles of antipyretic therapy // Russian Medical Journal. 2003, vol. 11, no. 1 (173), p. 31–37.
- Krasnova E.I., Chretien S.O. Streptococcal infection in children: modern approaches to anti-inflammatory therapy // Russian Bulletin of Perinatology and Pediatrics. 2010, No. 4, t. 55, p. 76–80.
E. I. Krasnova, Doctor of Medical Sciences, Professor S. O. Chretien A. V. Vasyunin, Doctor of Medical Sciences, Professor
NSMU, Novosibirsk
Contact information for authors for correspondence
special instructions
A history of erythroderma is not a contraindication for the use of Flemoxin Solutab®.
Cross-resistance with penicillin drugs and cephalosporins is possible.
As with other penicillin drugs, superinfection may develop.
The appearance of severe diarrhea, characteristic of pseudomembranous colitis, is an indication for discontinuation of the drug.
The drug should be prescribed to patients with infectious mononucleosis and lymphocytic leukemia with caution, because there is a high probability of the appearance of exanthema of non-allergic origin.