This article provides a detailed step-by-step description of anesthesia techniques using the STA (Single Tooth Anesthesia) system from Milestone Scientific (USA).
This article provides a detailed step-by-step description of the methods of anesthesia using the STA system (Single Tooth Anesthesia – anesthesia in the field of a single tooth), the company Milestone Scientific (USA).
The history of local anesthesia in dentistry goes back more than 150 years. However, it took decades for dentists to move from the Dr. Luer syringe and Novocaine to a disposable syringe with needles of different sizes and a solution of lidocaine as an anesthetic. It took several more decades to switch to the use of carpule syringes and carpule forms of anesthetics. However, despite all the innovations, the doctor was still limited to two traditional methods of pain relief: infiltration and conduction. The method of intraosseous anesthesia developed in recent years actually turned out to be quite traumatic and risky, since the anesthetic solution could enter the bloodstream, causing various complications, especially in patients at risk. Even intraligamentary anesthesia could not become a full-fledged replacement for mandibular anesthesia due to the short duration of pain relief, the complexity of implementation and the high incidence of side effects, such as injection pain, postoperative discomfort, soft tissue damage and bone resorption. All these side effects are mostly associated with the inability to control the speed of supply of the anesthetic solution, the accuracy of its administration and the pressure created in the tissues.
In recent years, computer technologies for supplying anesthetic solution have been increasingly developed. The ability to control the feed rate, which does not exceed the patient's pain threshold, the pressure created in the tissues and the position of the needle in the tissues, makes computer-controlled anesthesia the technique of choice in everyday dental practice. Modified intraligamentary anesthesia has made it possible to abandon conduction techniques in almost all clinical situations, thanks to the ability to accurately, computer-controlled injection of more anesthetic (which increases the duration of anesthesia) with minimal pressure (which reduces the number of postoperative complications) and low delivery speed (which makes the procedure virtually painless).
This article provides a detailed step-by-step description of anesthesia techniques using the STA (Single Tooth Anesthesia) system from Milestone Scientific (USA).
Milestone Scientific has developed a pre-injection technique that is highly recommended when using the STA System. The technique consists of three stages:
- Puncture
- preliminary anesthesia according to the type of topical anesthesia by pressing the cut of the needle with the tip of a cotton swab to the surface of the tissue with the supply of an anesthetic solution - Penetration
- slow two-way rotation of the needle as it moves through the tissue until it reaches the injection site - Injection of a standard amount of anesthetic solution
at a constant rate not exceeding the patient's pain threshold
This method provides a virtually painless injection for every patient.
All stages are easy to use and differ slightly from traditional techniques, however, the doctor is freed from the need to independently control the rate of anesthetic supply and pressure in the tissues - this function is performed by a computer, which greatly facilitates the procedure and makes it simpler and more predictable.
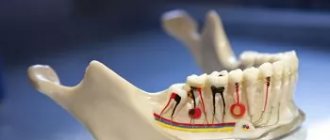
Conduction anesthesia of the nasopalatine nerve at the incisive foramen
The nasopalatine nerve, one of the nasal nerves of the nasal septum that innervates the nasal septum, begins at the sphenopalatine ganglion, passes along the nasal septum obliquely and medially forward, and enters the incisive canals on both sides of the nasal septum.
Both bone canals, starting on both sides of the nasal septum in the nasal cavity, passing through the hard palate, merge into one canal, which opens from the side of the oral cavity behind the central incisors with one hole - the incisive one.
Thus, both nasopalatine nerves exit through one opening - the incisive one, innervate the mucous membrane of the anterior part of the palate and anastomose with the branches of the anterior palatine nerves. Therefore, we are able to turn off the innervation of both nasopalatine nerves in the anterior part of the palate with one injection into the incisive foramen.
Location of the incisive foramen (target point). One line on which this opening is located is always accessible to us, this is the line of the median palatine suture, sutura palatina mediana. If we find such a definite and accessible line to us that would intersect the median palatal suture at the site of the incisive foramen, or if we know the distance of the incisive foramen at the indicated suture from some constant and accessible point to us, then our goal will be achieved and the location of the incisive foramen on the mucous membrane the shell of the sky was found. A constant perpendicular crossing the midpalatal suture at the site of the incisive foramen is the line connecting the distal edges of both upper canines. The constant points on the median palatal suture, the distances from which to the incisive foramen determine the location of the latter, are the place of contact of the upper central incisors and the edge of the alveolar process between them.
Our measurements of these distances on 75 adult and 30 children’s turtles gave the following results:
| Objects of research | Distance of the incisal foramen from the point of contact of the upper central incisors (in mm) | Distance of the incisal foramen from the alveolar edge between the upper central incisors (in mm) |
| Children | 9 | 5 |
| Adults | 10 | 8 |
Having several identification points, you can use others if some are missing.
Infiltration anesthesia
- Press the bevel of a 30G 1″ or 30G 1/2″ needle against the mucous membrane at the injection site and apply pressure using a cotton swab.
- Deliver anesthetic at a ControlFloTM rate of 8–9 sec.
- Slowly rotate the needle to pierce the first layer of soft tissue.
- Slowly advance the needle until the anesthetic injection site is reached.
- Once the needle reaches the site of injection of the anesthetic, perform an aspiration test. If blood is observed in the tip, reposition the needle and repeat the aspiration test.
- If the aspiration test is negative, continue injecting anesthetic at the ControlFloTM rate for 5 to 6 seconds, then change to the RapidFloTM rate and inject the usual amount of local anesthetic as if using a traditional carpule syringe.
- After injecting the recommended amount of anesthetic solution, wait 5-6 seconds and slowly remove the needle, trying to avoid any drops of anesthetic getting into the patient’s mouth.
- The effect occurs much faster than when using a traditional carpule syringe.
Technique for incisal anesthesia (injection site and needle direction)
Due to the fact that the lower jaw here, during incisal conduction anesthesia, prevents directing the needle perpendicular to the palate, during this injection we are forced to give the needle an oblique direction from the front and bottom, back and up.
The injection site, therefore, should be a point lying in front of the incisive foramen (or, rather, the projection of the incisive foramen onto the mucous membrane of the palate) by approximately 3-5 mm. When injecting at the greater palatine foramen, take a greater distance between the injection site and the place of projection of the greater palatine foramen onto the mucous membrane, because there the needle must go more obliquely.
This injection site is especially convenient because approximately here, in front of the incisive foramen, there is a constant elevation indicating the injection site. This elevation is the incisive papilla, papilla incisiva, located behind the space between the upper central incisors. Unfortunately, injections into the incisive papilla are very painful, since it is very rich in nerve endings,
Due to the excessive pain of the injection in this place, and also due to the fact that the anesthesia zone for this anesthetic injection is very small (the palatal surface of the mucous membrane within the four upper incisors), some authors completely unreasonably refuse to use this conduction anesthesia. To combat the strong sensitivity of the incisive papilla, it is recommended to lubricate it several times with a 5% solution of cocaine or 3% dicaine (it is best to “massage” the papilla with these solutions; in children, dicaine is used in a 2% solution). Sometimes we inject slightly in front or slightly to the side of the incisive papilla. It is clear that when injecting outside the incisive papilla, this deviation must be corrected by some change in the direction of the needle, i.e., when injecting in front of the incisive papilla, the needle should go more horizontally; when injecting a little laterally, to the right or left of the incisive papilla, we must give the needle, in addition to the direction from the front and bottom to back and up, also a slight deviation in the direction opposite to the injection site.
It is important to remember with this anesthetic injection that the solution should be released only after feeling the bone with the end of the needle. For this anesthesia, you can use a short, but strong and sharp needle, since the tissues in this place are very stubborn. The solution should be released in an amount of no more than 1/2 ml.
When the needle is advanced into the incisive canal by 8-10 mm, anesthesia occurs not only of the palatal mucous membrane within the four upper incisors, but to a large extent also of the named teeth themselves, due to the fact that the nasopalatine nerve, before exiting the incisive foramen, gives off nerve branches to It is recommended that these teeth enter the incisive canal carefully and no deeper than 10 mm; if this precaution is not observed, the needle can be penetrated into the nasal cavity. The entry of a needle into the nasal cavity is detected even before the release of the anesthetic solution - by minor bleeding from the nose, and after the solution is released - by its appearance from the nostril on the lip or through the choanae in the pharynx. Bleeding from the nose worries patients very much, although it does not pose any danger. Of course, if the needle gets into the nasal cavity and an anesthetic solution is released into it, no anesthesia will occur.
Experience in the clinical use of incisal conduction anesthesia in the treatment of maxillary incisors
A. V. Kuzin Ph.D., Central Research Institute of Dentistry and Maxillofacial Surgery
V.V. Voronkova Ph.D., Clinical Diagnostic Center of the First Moscow State Medical University named after I.M. Sechenov
M. V. Stafeeva , dentist (Moscow)
Often in clinical practice, a dentist is faced with difficulties in anesthetizing teeth and soft tissues in conditions of inflammation. To realize the pharmacological action of the anesthetic, it is necessary to hydrolyze it in tissue fluid, the pH of which is normally 7.3-7.4. During odontogenic inflammatory processes, the pH of the tissue fluid decreases to 6-6.5. Under such conditions, a significant part of the anesthetic molecules is not hydrolyzed.
Several basic principles of pain relief in inflammatory periapical processes can be identified. The anesthetic depot is created away from the site of inflammation. In this light, conduction methods of pain relief are preferable, which allow you to “bypass” the inflammatory infiltrate. A combination of several methods of injection anesthesia is used to achieve complete anesthesia.
In classical publications devoted to the issues of dental anesthesia, it has been proven that after incisal conduction anesthesia, anesthesia occurs not only in the soft tissues of the anterior 1/3 of the palate, but also in the pulp of the incisors [2, 4, 5, 10]. In foreign literature there is data on anesthesia of the pulp of central incisors in 55-58% and lateral incisors in 48-58% after incisal conduction anesthesia [8]. Some authors propose using incisal conduction anesthesia as an independent method of anesthetizing incisors using an automated syringe [10], however, this technique is ineffective and is not widely used. Other authors have proven the effectiveness of incisal conduction anesthesia, performed additionally when infiltration anesthesia is insufficient in the treatment of incisors [13].
In this article, we present experience in using a combination of infiltration anesthesia and incisal conduction anesthesia for anesthesia of maxillary incisors in conditions of inflammation.
Features of the innervation of the maxillary incisors. The incisors of the upper jaw are innervated by the anterior superior alveolar nerves (ASN) Nn alveolaris superiors anteriores (trigeminal nerve system, maxillary nerve). The PVANs are part of the superior dental plexus, formed by the anterior, middle and posterior alveolar nerves. The PVANs pass through the infraorbital canal, in the middle of which they shift medially towards the bottom of the nasal cavity and move to the apices of the roots of the canines and incisors. Alveolar canals containing PVAN are clearly visible on cone-beam tomography [7, 9, 14], their diameter is 1.25–2.5 mm. In foreign literature, these canals are called convoluted (canalis sinuosis) [14, 15, 18]. According to international anatomical terminology, there is no such term as “convoluted canal”: all microcanals of the upper jaw that approach the roots of the teeth are called alveolar (Canalis alveolaris) [3].
In the anterior part of the upper jaw, cross innervation of the incisors is observed. The PVANs of the right and left sides form a dense network of anastomoses going to the incisors and canines [4, 5, 16, 11]. In the area of the central incisors, the PVAN is anastomosed with the nasopalatine nerve [7]. In the literature, there is anatomical evidence of the innervation of the maxillary incisors by the incisive nerve [17].
Study of the topography of the incisive canal. In order to study the intraosseous anatomy of the incisive canal and anterior superior alveolar canals, we studied archival data from cone-beam tomography of 60 patients (PaX-i3D Vatech tomograph). Using the supplied software, serial sections were made in the area of the incisive canal and alveolar canals of the anterior superior alveolar nerves.
Based on the results of the study, the following features of the anatomy and topography of the incisive canal were identified. The incisive canal begins with two separate nasopalatine openings, which open in the anterior part of the floor of the nasal cavity on both sides of the nasal septum (Fig. 1). The two canals are then combined into one incisal canal, giving it a Y-shape. The incisive foramen is located 8-10 mm posterior to the central incisors of the upper jaw, immediately below the incisive papilla. The length of the incisive canal is on average 8–9.5 mm, according to our data, which is comparable with the data of other authors [13].
We have identified several forms of the incisive canal (Fig. 2). In most cases, the canal has an hourglass shape; less commonly, a funnel or cylinder shape is found. In one case, two incisive canals located parallel in the sagittal plane were identified. It is worth noting that in the literature there are descriptions of 4 to 6 forms of the incisive canal, representing extreme forms of anatomy [7, 9].
In our opinion, the depth of needle advancement into the incisive canal directly affects the onset of anesthesia of the incisor pulp. We measured the possible depth of needle advancement into the incisive canal (Fig. 2). The length from the apex of the incisive papilla to the narrowing of the incisive canal is 9-12.5 mm (average 10.6 mm). Thus, when performing incisal conduction anesthesia, we recommend advancing the needle into the incisive canal 8-10 mm or until it contacts the bone tissue.
rice. 1 pic. 2a Fig. 2b Fig. 2c Fig. 2g
In half of the cases (56%) we detected a relationship between the alveolar canals and the incisive canal using CBCT. At the level of the bottom of the nose, the PVAN microchannels were connected to the incisive canal. In some cases, the microcanals were not connected to the incisive canal and had an independent course in the thickness of the alveolar process, opening onto the area of the anterior 1/3 of the palate with independent openings. Foreign authors call these “additional” holes “foramina Scarpa” [18]. We identified these holes in 26% of cases.
These features of the incisive canal explain the diffusion path of the anesthetic from the incisive canal to the apexes of the incisor roots. When performing incisal conduction anesthesia, the anesthetic is distributed deep into the incisive canal to the bottom of the nasal cavity, and through anastomoses to the anterior dental plexus to the apexes of the incisor roots (Fig. 3).
Study of the clinical effectiveness of incisal conduction anesthesia. We have studied the effectiveness of anesthesia for maxillary incisors under conditions of inflammation. Outpatient treatment was carried out for 120 patients diagnosed with periapical abscess without fistula; suppuration of a radicular cyst."
To anesthetize the central or lateral incisor from the side of the causative tooth, infiltration anesthesia was performed in the area of the maxillary canine, where an anesthetic depot of 0.8-1.2 ml was created (Ubistezin Forte). Using this method, we created an anesthetic depot away from the source of inflammation and blocked the anterior superior alveolar nerves.
After infiltration anesthesia, an additional incisal conduction anesthesia of 0.6 ml (Ubistezin forte) was performed. Due to the distribution of the anesthetic up the incisive canal, the incisive nerve and anastomoses of the anterior superior alveolar nerves on the opposite side are blocked.
We performed incisal conduction anesthesia in 3 stages (Fig. 4). Previously, topical anesthesia of the incisive papilla was performed using a cotton swab soaked in topical anesthetic. After 5 minutes, infiltration anesthesia of the incisive papilla was performed. To reduce the degree of pain of the injection, a needle was inserted on the side of the incisive papilla, and the anesthetic was slowly injected until signs of ischemia appeared (approximately 0.1 ml). The third stage was incisal conduction anesthesia according to the generally accepted method: a needle was inserted into the incisive papilla, the needle was moved upward and backward parallel to the axis of the central incisor. The needle was advanced (30G (0.3 mm), 27G (0.4 mm) X 16 mm) to a depth of 8-10 mm or until the needle tip came into contact with the bone tissue. An anesthetic depot of 0.5 ml was slowly created.
rice. 3a Fig. 3b Fig. 4a Fig. 4b Fig. 4v
Carrying out incisal conduction anesthesia in three stages can reduce the pain of the injection. The speed of administration of the anesthetic plays an important role in reducing pain. According to international standards EFAD and IFDAS, the rate of introduction of the anesthetic solution into the tissue should be 1 ml/min. Based on these data, we introduced 0.6 ml of solution into the incisive canal in 30-40 seconds.
For anesthesia, we used a standard carpule syringe and an automated injector (TSD SleeperOne), which greatly facilitates control over the rate of injection of the anesthetic solution [5, 6]. The program built into the device regulates the supply of anesthetic: at the initial stage, the anesthetic is supplied dropwise at a rate of 1 drop/sec., then the anesthetic is supplied at a rate of 1 ml/min.
To assess the effectiveness of the pain relief performed, we used the S. T. Sokhov scale (1982) [1]. All patients underwent therapeutic dental treatment; according to indications, a releasing incision was performed, the periapical lesion was opened, and its drainage was performed. According to our data, the combination of anesthesia used made it possible to carry out the interventions completely painlessly in 85% of cases. In 9% of cases, patients experienced mild pain and there was no need for additional pain relief. In 6% of cases, endodontic treatment was painless, but during the incision the patient felt severe pain, which required repeated anesthesia. To achieve complete anesthesia, infiltration anesthesia (0.6 ml) was performed at the site of greatest pain.
At the stage of dynamic observation of patients, we assessed possible post-injection complications from incisal conduction anesthesia. Most patients immediately after incisal conduction anesthesia experienced a feeling of numbness in the nose (its internal part). Subsequently, before performing anesthesia, we always warned patients about the possible appearance of nasal numbness after anesthesia. When re-examined on the third day, half of the patients noted discomfort in the area of the incisive papilla: “sensation of a tubercle”, “swelling of the palate” (during an external examination, no signs of swelling or inflammation were detected). On the seventh day, when questioning the patients and examining them, no complaints or complications were identified. According to our observations, we did not identify cases of sensory impairment in the area of the anterior 1/3 of the palate in patients. We also did not detect signs of neuritis: the patients did not notice pain in the bottom of the nose, the anterior part of the hard palate, or irradiation of pain. We believe that injury to the incisive nerve certainly occurs during incisive conduction anesthesia, but it is minimal due to the fact that the incisive canal contains, in addition to nerve structures, vessels, adipose tissue, connective tissue, and minor salivary glands [12] . With slow administration of the anesthetic (1 ml/min), its distribution occurs gradually without pronounced compression of the nerve structures, which is confirmed by the absence of post-injection neurological complications in our patients. Also, modern carpule needles have a small diameter and an atraumatic bevel, which also reduces the degree of tissue injury.
Conclusion. Currently, when anesthetizing the teeth of the upper jaw, infiltration anesthesia is indicated in most cases. Anesthesia from the palate is carried out during surgical interventions (tooth extraction surgery). We believe that incisal conduction anesthesia (0.6 ml of 4% articaine with epinephrine “Ubistezin Forte”) is indicated for the therapeutic and surgical treatment of maxillary incisors in conditions of inflammation of the periapical tissues. The use of a combination of infiltration anesthesia from the outside and incisal conduction anesthesia allows for complete anesthesia of periapical tissues.
List of abbreviations
PVAN - anterior superior alveolar nerves
CBCT - cone beam computed tomography
Complications of incisal anesthesia
- Vascular injury. Vascular injury during this anesthesia is a very common occurrence, since the incisive foramen, like the greater palatine foramen, is surrounded by a dense fan of blood and lymphatic vessels and contains branches of the pterygopalatine artery. Bleeding from the injection site is insignificant and soon stops spontaneously or by pressing the bleeding area with a sterile gauze pad.
- The appearance of ischemic areas of facial skin.
The appearance of ischemic areas of facial skin during incisive conduction anesthesia deserves attention. The vessels passing through the incisive foramen are, as is known, in close connection with the vessels of the pterygopalatine fossa; the vessels of the latter also supply blood (in addition to other tissues) to the skin of the face. If an anesthetic solution, when wounding one of the vessels passing through the incisive canal, enters the pterygopalatine fossa and causes contraction of a vessel there, then ischemia occurs in one or another area of the facial skin, depending on which particular vessel has undergone narrowing. Ischemic areas during anesthetic injections at the incisive foramen can appear not only on one half of the face, as happens with anesthetic injections at the greater palatine foramen, but also on both halves of the face simultaneously. In the latter case, they can be symmetrical or asymmetrical. The occurrence of such bilateral ischemia is explained by the fact that the incisive foramen communicates equally with both pterygopalatine fossae, while the greater palatine foramen communicates only with the pterygopalatine fossa of the corresponding side. When injected at the incisive foramen, the anesthetic solution can immediately enter both pterygopalatine fossae and narrow the same or different vessels there. - Entry of the needle into the nasal cavity.
In order to prevent this complication, you should not go deeper into the incisive canal by more than 8-10 mm during incisal conduction anesthesia. If a needle gets into the nasal cavity, do not release the anesthetic solution. To re-inject, it is necessary to replace the infected needle in the nasal cavity with another, sterile one.
Anesthesia of the anterior and middle superior alveolar nerve
- Place a 30G 1/2″ needle at the injection site, centered between the midpalatal suture and the free edge of the gum on a line drawn between the premolars.
- Press the bevel of the needle against the mucous membrane and press on it with a cotton swab.
- Deliver anesthetic at a ControlFloTM rate of 8–9 sec.
- Slowly rotate the needle left and right to penetrate the first layer of soft tissue.
- Slowly advance the needle while continuing to rotate it.
- Once bone contact is achieved, position the needle at a 90° angle to the premolars and perform an aspiration test.
- If blood is observed in the tip, reposition the needle and repeat the aspiration test.
- If the aspiration test is negative, continue injecting anesthetic at the ControlFloTM rate. Inject the usual amount of local anesthetic as if using a traditional carpule syringe: when using 4% articaine with 1:200,000 epinephrine, the recommended dosage for adults is approximately 1/3 to 1/2 a carpule.
- After injecting the required volume of anesthetic, wait 6 s and slowly withdraw the needle using a bilateral rotation technique, taking care to avoid dripping anesthetic into the patient's mouth (Fig. 1).
The area of anesthesia extends from the medial buccal root of the first molar to the central incisor, including the palatal tissue in this quadrant.
Rice. 1
AMSA (Anterior Middle Superior Alveolar) technique
Mandibular anesthesia
Press the bevel of a 27G 11/4″ needle against the mucosa at the injection site and apply pressure using a Q-tip.
- Deliver anesthetic at a ControlFloTM rate of 8–9 sec.
- Slowly rotate the needle to pierce the first layer of soft tissue.
- Using a bilateral rotation technique (rotating the needle left and right up to 180° to prevent deflection during penetration), slowly advance the needle until it reaches the anesthetic injection site while delivering the solution at ControlFloTM speed.
- Once the needle reaches the injection site, perform an aspiration test (release the pedal or, if the cruise control function is activated, press the pedal) to ensure that the tip of the needle is not in the lumen of a large blood vessel. If blood is observed in the tip, reposition the needle and repeat the aspiration test.
- If the aspiration test is negative, continue injecting anesthetic at the ControlFloTM rate for 5 to 6 seconds, then change to the RapidFloTM rate and inject the usual amount of local anesthetic as if using a traditional carpule syringe.
- After injecting the recommended amount of anesthetic solution, wait 5-6 seconds and slowly withdraw the needle using a bilateral rotation technique, trying to avoid dripping the anesthetic into the patient's mouth (Fig. 3).
If repeat injection is necessary, TurboFloTM speed can be used.
Rice. 3
Mandibular anesthesia