Dental cements
Depending on the composition and purpose, dental cements are divided into the following types:
- 1) zinc phosphate (phosphate);
- 2) silicate;
- 3) silicophosphate;
- 4) silver and copper cements;
- 5) zinc oxide eugenols;
- 6) zinc sulfate.
Zinc phosphate cements . Zinc phosphate cement is a hydraulic binder consisting of separately stored powder and liquid. The first phosphate cement was created in 1832 by Ostermann. The powder contained calcium oxide CaO, and the liquid contained phosphoric acid. Thus, the first phosphate cement was calcium phosphate. The first successful composition of zinc phosphate cement was developed: in the USA by Ward in 1880. Ward cement powder contained 81% zinc oxide and 19%) aluminosilicate, and the liquid consisted of phosphoric acid, sodium phosphate and water. Modern cements were created at the end of the last century, and their formulations have not changed significantly.
Compound. Phosphate cement powder is a product of fine grinding of frit obtained by sintering a multicomponent mixture of oxides and salts (ZnO, CaO, SiO2, MgCO3, etc.). These components make up approximately 97.5% of the total mass of the charge. As a mineralizer, up to 2% of the mass of the cryolite charge and a small amount of additives that modify the properties of cement (Bi2O3, CaF2, BaCrO3, Al2O3, etc.) are added to the mixture. Approximate composition of phosphate cement powder: 75-90% ZnO, 5-13% MgO, 0.05-5% SiO2, 0.05-2.5% R2O3 (Table 75). Zinc phosphate cements differ from each other mainly in the composition of the powders. The main component of all powders is zinc oxide. The modifying magnesium oxide contained in the powder is approximately 10 times less than zinc oxide. The cement should not contain soluble arsenic compounds, which can have a harmful effect on the pulp. The powder must pass through a sieve with 10,000 holes per 1 cm2.
Liquid phosphate cement is an aqueous solution of orthophosphoric acid containing zinc, aluminum and magnesium phosphates. The liquid is prepared by partial neutralization of an aqueous solution of phosphoric acid with hydrates of the oxides of these metals. Zinc, aluminum and magnesium phosphates are added to the liquid as buffers to reduce the rate of chemical interaction between the liquid and the powder. Thus, they are regulators of the rate of setting of cement. A number of researchers believe that the optimal water content in phosphate cement liquid should be 33±5%. The water content regulates the degree of dissociation of electrolytes in the liquid. When the electrolyte is diluted, the degree of dissociation increases, which accelerates the setting process of cement. Despite the fact that phosphate cement powders are similar in composition, you cannot use a liquid of a different brand of cement. Approximate composition of phosphate cement liquid: P2O5 39±45%, ZnO 8±12%, Al2O3 3±6%, H2O 37±50% (Table 76).
Properties. Mixing the powder with the liquid is carried out on a thick, smooth glass plate using a chrome-plated or nickel-plated spatula. A spatula with a damaged coating is unusable. The optimal ratio of powder and liquid is indicated in the instructions and for different brands of phosphate cements it ranges from 1.8 to 2.2 g of powder per 0.5 ml of liquid. Normal temperature conditions for mixing are considered to be 18-20 °C. At temperatures above 25 °C, it is recommended to cool the plate, and when working in a cold room (16 °C), take a small excess of powder. To obtain a homogeneous cement mass, the following technique for its preparation is recommended. The required amount of liquid and powder is applied to the glass plate with a pipette (Fig. 68).
Then use a spatula to divide the powder into six parts, as shown in Fig. 68. Each 1st, 2nd and 3rd parts constitute 1/4 of the total amount of powder, the 4th - 1/8, and the 5th and 6th - 1/16. The mixing time should not exceed 1.5 minutes and is distributed as follows:
The consistency of the molding mass is considered normal if, when the spatula is separated from the mass, it does not follow it, but breaks off, forming teeth up to 1 mm high. If the mass turns out to be thick, then it is impossible to adjust its consistency by adding liquid. In this case, you need to prepare a new portion.
The setting time of zinc phosphate cement can be adjusted by changing the temperature of the glass plate on which the mixing is carried out, the amount of liquid and the mixing speed. By heating the glass plate, you can speed up the setting of the material, and by cooling, you can lengthen it. It must be borne in mind that if the plate is cooled to a low temperature, water vapor from the air may condense on it, and this can lead to accelerated setting of the cement mass. When mixing with a thick consistency, setting proceeds faster and the cement is stronger. By gradually adding powder to the liquid slowly, the curing time increases. To obtain a homogeneous mixture and a structuring product of maximum strength, it is necessary to add the powder to the liquid in small portions at certain short intervals, as described above. The setting time must be adjusted quite precisely. During rapid hardening, the resulting crystals are destroyed during the mixing process and the structuring product turns out to be fragile and rough. The optimal setting time is 4-10 minutes.
The following factors influence the setting time:
- 1) composition and sintering temperature of powder components (preparation of frit);
- 2) composition of the liquid (content of water and buffer salts);
- 3) degree of dispersion of the powder (the finer the powder, the faster the setting occurs);
- 4) temperature;
- 5) technology for mixing powder with liquid (the slower the powder is added, the longer the setting);
- 6) the greater the liquid/powder ratio, the slower the setting;
- 7) prolonged mixing extends the setting time.
Adjusting the curing time by increasing the relative amount of liquid should be avoided, as this reduces the strength of the cement and increases its solubility.
The consistency of the initial powder-liquid mixture is important. The thick consistency of the initial mixture after curing forms cement with high physical properties. The consistency of the molding mass should be selected depending on the clinical application. Thus, when strengthening an inlay in a prepared cavity, a thick mixture cannot be recommended, since it does not have high fluidity and the inlay will not be accurately strengthened in the cavity. Therefore, a liquid mixture is used to cement crowns, bridges, and inlays. It must be borne in mind that each brand of cement requires a certain powder/liquid ratio to obtain a standard consistency. In order for the inlay or crown to be accurately installed and secured, the cement film must be sufficiently strong and thin. The minimum film thickness is related to the size of the powder particles. However, the film may be thinner than the maximum particle size, since their diameter is reduced due to dissolution in acid when the powder is mixed with liquid. C. W. Skinner (1955) determined that cement from a powder with a particle size of 75 microns gives a film thickness of 35 microns. According to the ISO standard, the film thickness should be no more than 40 microns at a specific pressure on the molding compound of standard consistency of 0.75 MN/m2 for 10 minutes.
Strength, solubility, shrinkage . Domestic and international standards for zinc phosphate cements impose the following requirements: the consistency of the mixture must be such that the disc is 30+1 mm in diameter, curing time at 37 ° C is at least 4, maximum 10 minutes, compressive strength after 7 days is at least 84 MN /m2, film thickness - maximum 40 microns, solubility - maximum 0.2%, arsenic content - maximum 0.00029%.
Thickly mixed cements have maximum strength. However, the thick mixture is inconvenient to work with, so the consistency is selected depending on the type of work. Cement acquires the greatest strength corresponding to a given batch after 24 hours, but after 1 hour its strength is approximately 2/3 of this value. The strength of zinc phosphate cements decreases over time due to the partial solubility of the matrix in saliva. Thickly mixed cement has a smaller relative amount of matrix phase and is therefore less susceptible to dissolution and disintegration. The contact of saliva with cement during its setting reduces its strength and increases solubility. Zinc phosphate cements exhibit noticeable shrinkage, which, however, is less pronounced due to the thinness of the cement layer. For example, if the film under the insert has a thickness of 0.1 mm and if the cement gives a linear shrinkage of 0.08%, then the film shrinkage will be only 0.00008 mm, or 0.08 microns. This shrinkage is not clinically significant. In table 77 shows the main indicators of some brands of phosphate cements produced by the domestic medical industry and foreign companies.
The connection of zinc phosphate cement with dental tissues, metals and other materials is not due to adhesion, but to surface roughness. When cementing, the surface of a metal structure must be roughened by treating it with sandpaper or carborundum stone. Powder of domestic brands of phosphate cement, which contains zinc oxide and magnesium oxide, does not cause pulp irritation.
Investigating phosphate cement of the Dentachem brand, the powder of which has the greatest deviation in composition from generally accepted formulations, Lange (1964) came to the conclusion that the irritating effect of this cement on the pulp is due to the composition of the powder (SiO2 content 12.55% instead of 0.05 ± 5% , and Al2O3, which is usually absent, is 9.58%). Most researchers attribute the irritating effect of phosphate cements to orthophosphoric acid, which did not react during the process of mixing and structuring the molding mass. At the initial stage of mixing, the acidity of the mass is high and the pH reaches 1.6. During the structuring reaction, the pH increases rapidly and approaches 7.0 by the end of hardening. E. Dolder (1955) found that phosphate cement becomes neutral on the 2nd day after mixing. D. Hattyasy (1961) observed an acidic reaction of the liquid consistency of the molding mass for only 2 hours, and for a thick batch - only for 5 minutes. Most researchers believe that fluctuations in acidity during the setting process are within physiological norms.
Indications for use. Zinc phosphate cements are used:
- 1) as an insulating lining when filling teeth with other materials - amalgams, silicate and silicophosphate cements;
- 2) for permanent fillings in case of covering a tooth with an artificial crown;
- 3) for temporary fillings with an extended service life;
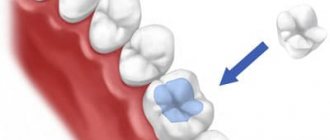
- 4) for fixation of fixed structures: bridges, facets, inlays, crowns and pin teeth.
Phosphate cement is introduced into the prepared cavity in small portions with thorough condensation to all the walls of the cavity. The molding paste at the time of its introduction into the cavity must be in a sufficiently plastic state, which ensures the adhesion of cement to the walls of the cavity and improves the quality of the filling. When applying the lining, the consistency of the molding mass should be such that the permanent filling material is somewhat embedded in the phosphate cement, but at the same time it should not be squeezed out of the tooth cavity and protrude to the surface. When using phosphate cement, you cannot dry the tooth cavity with ethyl alcohol, as this causes dehydration of dentin and, as a result, penetration of acid from the cement into the depths of the tooth, which can damage the pulp. It is recommended to dry the cavity with cotton wool and then with air. During the filling process, care must be taken to ensure that no saliva gets on the surface of the filling. During the hardening time (2 hours), the filling is covered with dental varnish or a layer of molten wax.
Copper and silver cements . Copper and silver cements are phosphate cements modified with bactericidal substances, used for filling baby teeth. Bactericidal substances (CuO, Cu2O, AgCI, Cul, etc.) are added to the powder composition. Copper and silver cements produced by foreign companies have different colors depending on the bactericidal substance introduced. Thus, copper oxide (CuO) colors cement black (“black copper cement”), cuprous oxide gives cement a red color (“red copper cement”), silicon copper Cu2SiO2 gives it green (“green copper cement”). Colorless compounds of copper and silver (CuCl, Cul, Ag3PO4, CuSiO3, Cu2I2) do not change the color of cement. The aesthetic properties of these cements are low. The surface of this group of cements is hard but easy to process. The disadvantage of copper and silver fillings is their instability. They are quickly washed out of the tooth cavity and must be restored. Copper and silver cements are now rarely used and, presumably, they have lost their importance in dentistry.
Silicate cements . Silicate cement is a hydraulic binder. Like zinc phosphate cements, silicate cements are produced by industry in the form of a powder-liquid set. To imitate tooth tissue, cements are produced in several shades, which are designated by numbers. Silicate cements have high aesthetic properties. Fillings made from them have a shine characteristic of natural tooth enamel. In color and transparency, they reproduce tooth tissue well and are used mainly for filling anterior teeth. Industrially produced silicate cements must meet the requirements of the standard given in Table. 78.
Compound. Silicate cement powder is a finely ground sintering product of a multicomponent mixture, the main ingredient of which is silicon oxide. Silicate cement liquid is an aqueous solution of phosphoric acid containing zinc, aluminum and sometimes magnesium phosphates. Approximate composition of silicate cement (percentage by weight):
To increase the durability of cement and intensify silicate formation, small amounts of ZnO, B2O3, MgO, etc. can be added to the charge. Silicate cement powder in its chemical composition differs from phosphate cement powder and is a finely ground glass consisting of aluminosilicates, fluoride compounds and pigments, creating a range of shades to imitate tooth tissue. High quality cement powders contain a large amount of fluoride compounds (up to 15%). It is believed that the supposed anti-cariogenic properties of silicate cements are associated with such a high content of fluoride compounds. Silicate cements containing fluoride compounds reduce the solubility of the enamel adjacent to the filling. In addition, the presence of fluorides in the powder reduces the solubility of the filling in water. The degree of powder dispersion is controlled by sifting through a sieve with 10,000 holes/cm2. The residue on the sieve should not exceed 0.5%.
The composition of the liquid of silicate cement is close to the hardening liquid of phosphate cements, but one cannot be replaced by the other. The water content in it ranges from 40+5%, which is on average 7% higher than the water content in the liquid of zinc phosphate cements. The pH of the liquid is approximately 2.0. A significant part of the acid is neutralized by metal oxides. To avoid crystallization of the salt (phosphate), neutralization is carried out with various oxides (ZnO, Al2O3, MgO) in such a way that the concentration of each salt does not reach a saturation state.
Depending on the relative humidity of the air, the liquid of silicate cements either absorbs water vapor from the air or loses some of the water due to evaporation. In an atmosphere with a relative humidity of 80%, water evaporates quite quickly, which leads to a change in the composition of the liquid. The appearance of crystals on the neck of the bottle means a large loss of water, resulting in crystallization. The remaining liquid after using all the powder is not recommended for mixing the powder of a new set of cement. To avoid changes in the composition of the liquid, the bottle should not be left open. To prevent water evaporation, sometimes a little Vaseline oil is added to the bottle of liquid, which forms a thin protective layer on the surface of the liquid. The density of the liquid ranges from 1.56 to 1.66 g/cm3.
Properties. The preparation of silicate molding mass has its own characteristics, the observance of which determines the quality of the filling. Silicate powder has abrasive properties and can remove metal particles from a stainless steel spatula, which leads to discoloration of the filling and negatively affects the curing process. Mixing should be done on a smooth glass plate or in an agate mortar using a plastic, bone, cobalt-chrome or agate spatula. The optimal mixing temperature is 18-20 °C. At temperatures above 22 °C, it is advisable to cool the glass plate, and when the temperature drops to 16 °C, slightly more powder is introduced. The optimal ratio of powder and liquid is indicated in the cement manufacturer's instructions and varies for different brands of cement from 1.35 to 1.55 g of powder per 0.4 ml of liquid.
All manipulations with cement must be stopped immediately after the start of hardening, i.e., the formation of a gel. If the gel is destroyed, the filling will have poor mechanical and physical properties. Mixing must be carried out by introducing the powder into the liquid in large portions so that the reaction between silicon oxide and phosphate acid begins simultaneously. When adding powder in small portions, the first portions begin to set before the subsequent portions are added and the gel of the first portions is destroyed. Mixing must be completed before the molding mass begins to set, i.e. within 1 minute. Mixing time is distributed as follows:
Before adding each new portion, the cement mass must be homogeneous. The technique for mixing cement is shown in Fig. 69. At the final stage, mixing must be carried out with some force in order to remove air from the powder. There are recommendations to prepare the molding compound using a mechanical amalgam mixer. The cement mass becomes homogeneous after 10-20 s.
The setting speed of silicate cements is influenced by the following factors:
- 1) degree of powder dispersion;
- 2) acid concentration;
- 3) temperature;
- 4) consistency of the batch;
- 5) mixing time.
The setting time of cement depends on the ratio of SiO2: (Al2O3 + CaO)2. The smaller it is, the faster the molding mass cures, with the composition of the remaining components being constant. If the cement sets quickly, then the gel phase may form before the cement is introduced into the tooth cavity and, as a result of the destruction of the gel during further work, the filling will be weakened. Powder with a high degree of dispersion has a more developed surface, so it reacts faster with the cement liquid. Prolonged mixing prolongs the setting time due to the destruction of the gel fraction. The optimal setting time established by the standard for silicate cement: beginning - 3 minutes, end - 8 minutes. In clinical practice, it is recommended to influence the setting speed by changing only the temperature of the glass plate for mixing cement, since the influence of other factors entails a deterioration in the properties of the structuring product - cement.
In order for the cement mass to be well molded, have a given viability and be structured into durable cement, it must be prepared to a certain consistency. It must be borne in mind that the standard consistency (normal thickness of the batch) is obtained by mixing 0.4 ml of liquid for silicate cement and 0.5 ml for phosphate cement with such an amount of powder that 0.5 ml of the mixture forms a disk with a diameter of 25 mm at compression of the mass between two glass plates under a load of 25 N, installed mainly for quality control and comparative studies of cements of various brands. The standard consistency is not optimal for every brand of cement.
The main disadvantage of silicate fillings is their relatively high solubility in the oral cavity. Currently produced silicate cements have a solubility of about 0.6-0.8% after 24 hours at 37 °C in distilled water, although the ISO standard allows their solubility to be up to 1%. Like phosphate cements, the solubility of silicate cements proceeds through the disintegration mechanism. Phosphate cements are less soluble in water than silicate cements, but silicate cements are more stable in oral conditions.
Moisture entering the cement during its hardening causes swelling of the gel fraction. At the same time, a certain amount of phosphoric acid is washed out and the filling becomes even more soluble. It is necessary to protect the filling from moisture only until it sets. To minimize the solubility of a silicon cement filling, it must be isolated from contact with moisture for 3 hours from the moment of manufacture. The best insulating effect is achieved by using alkali celluloid varnish, petroleum jelly and wax. Thus, the solubility of silicine at 37 °C is 1.21 ± 0.03% of the mass of the sample that was in contact with moisture during the setting period, and when insulated with alkali-celluloid varnish it is only 0.14 ± 0.02%.
All silicate materials shrink, the external manifestation of which can be a dark line that often forms over time around the filling (contamination of the gap between the filling and the wall of the tooth cavity). The presence of fluorides in silicate cements reduces the possibility of secondary caries. Linear shrinkage of silicate cements of various brands after 7 days of curing ranges from 0.15-0.5%. Moisture helps stabilize the size of silicate cements. Consequently, the drying of silicate fillings can negatively affect the quality of their marginal adhesion to the walls of the oral cavity. The amount of shrinkage depends on the powder/liquid ratio. As this ratio increases, shrinkage decreases.
The hardness of silicate cements varies widely, which is due to the heterogeneity of the composition of the material. Over time, the hardness of cements increases slightly. It increases rapidly in the first 3 hours. This process continues for a week. Silicate cements have greater compressive strength than phosphate cements. The maximum change in the transparency of cements, which determines the high aesthetic quality of the material, occurs after 24 hours. The light absorption established by the standard should be 35-55%, i.e. 45-65% of the light should pass through the tested cement sample. In table 79 shows the main physical and mechanical characteristics of silicate cements.
Silicate cements do not exhibit true adhesion to tooth tissue. The observed connection of the filling with enamel and dentin is determined by retention and surface roughness. According to V.V. Mitina, this relationship is approximately 1.9±0.2 MN/m2. Irritation of the pulp by silicate cement has been discovered since the introduction of the first Ascher “artificial enamel” cement into dental practice. It is generally accepted that the main reason for the irritating effect on the pulp is the acidic reaction of silicate cements. In this regard, a number of researchers have carried out work to determine the change in pH during the structuring of cement and the change in pH over time. If for phosphate cements the pH is 7.0 on the 2nd day after mixing, then for silicate cements the pH gradually changes from 4.0 during mixing to 7.0 within 30 days. To exclude possible necrosis of the pulp by arsenic, the International Standard provides for a maximum arsenic content in cement of 0.0002%.
Indications for use. Silicate cement is used for filling frontal and lateral teeth in the vestibular localization of cavities, when the filling must meet aesthetic requirements. A high-quality filling can only be obtained if all sealing conditions arising from the properties of this material are strictly observed. It is advisable to introduce silicate cement into the tooth cavity in one portion, since repeated injection of the cement mass does not ensure the integrity of the filling. The consistency of the cement mass should be such that, with light pressure from the spatula, the surface of the cement acquires a shiny (wet) appearance. When the spatula is torn off, the mass should not be pulled behind it by more than 2 mm. To obtain a good filling surface, it is recommended not to condense the material with a plugger, but to press a cellophane or celluloid plate lightly lubricated with Vaseline. The plate is inserted with a sliding motion, ensuring a smooth surface of the vestibular surface of the filling.
The filling molding procedure must be completed before the cement begins to set. Modeling the filling during the setting period of the cement leads to the formation of a network of cracks on its surface. This filling has increased solubility and poor color fastness. In this regard, all excess cement mass that was not removed during the treatment of the filling with a celluloid strip is removed with a carborundum head after the final setting of the cement (2-3 hours). While the cement hardens, the filling is isolated from contact with moisture, as mentioned above.
Silicophosphate cements . Compound. Silicophosphate cements are silicate cements modified with zinc phosphate cements, and in their chemical and physical properties they occupy an intermediate position between them. This type of cement includes erkadont and silidont, produced by the medical industry. Erkadont is a mixture of 60% silicate cement and 40% phosphate cement, and Silidont is a mixture of 80% silicine and 20% phosphate cement. Approximate composition of silicophosphate cements:
Properties. Mixing silicate phosphate cement is done in approximately the same way as silicate cement. This cement is easier to mix than silicate cement. It is recommended to add smaller portions of powder so that the process of chemical interaction between the components of the mixture occurs more fully. The structuring product of the molding mass is a conglomerate of silicic acid gel and a crystalline mass of the curing products of phosphate cement. Silicophosphate cement fillings are radiopaque. During the setting process, the pH of the cement mass increases. On the cement surface, after 4 hours, the pH rises to 6.0 (the pH of phosphate cements reaches this level after 1 hour). Fillings made from silicate phosphate cements are less brittle than silicate ones. In table 80 shows the main characteristics of silicophosphate cements.
According to V.I. Mitina, the microhardness of silydont, depending on the curing conditions, ranges from 327+52 to 1004+24 MN/m2 (at 37°C and 100% humidity).
Indications for use. Silicophosphate cements are used for filling anterior teeth in cases where cavities are localized when it is not necessary to satisfy aesthetic requirements, i.e., when the cavities do not extend to the vestibular surface of the tooth. Their lower fragility compared to silicate cements allows them to be used instead of amalgams for filling lateral teeth with chewing-proximal cavities (with thinned tooth walls and when amalgam is undesirable for aesthetic reasons). Silicophosphate molding mass is introduced into the cavity in several portions with thorough condensation to the walls. During the setting period (2-3 hours), the filling is isolated from contact with saliva with molten wax, acrylate varnish or Vaseline.
Glass ionomer cements . The disadvantages inherent in silicate cements—high solubility, lack of adhesion to tooth tissue—led to the creation of a new group of dental cements. The first glass ionomer cement ASPA was released (USA) in 1971. The new type of cement compares favorably with silicate cements in many respects. Glass ionomer cements have a good bond with enamel and dentin, do not dissolve in oral fluid, have high abrasion resistance, are colorfast and adhesive to gold, platinum and oxidized foil.
Compound. Glass ionomer cements are powder-liquid materials. The powder is finely ground aluminosilicate glass. This glass is made with a high content of calcium and fluorine and a small amount of sodium and phosphates. The fusion of components is carried out at a temperature of 1150–1300 °C. At 1150°C the quality of the powder is better. After crushing, the glass mass is subjected to fine grinding. The best powder is obtained by grinding in air-jet grinding units, which absolutely exclude contamination of the powder with other substances. The particle size of the powder must be less than 42 microns. The liquid is an aqueous solution of polyacrylic acid having an average molecular weight of 23,000.
The properties of polyacrylic acid are considered in the description of polycarboxylate cements. To obtain durable ionomer cement, a high concentration of polyacrylic acid, which is poorly soluble in water, is required. First, a 20% acid solution is prepared, and then it is evaporated under vacuum until approximately 50% viscous solution is obtained.
The molding mass obtained by mixing powder with liquid hardens as a result of a series of competing chemical reactions. The structuring of ionomer cement is due to the formation of a complex combined matrix of silicate and polyacrylate matrices. The silicate matrix arises from silica gel formed as a result of the interaction of polyacrylic acid with the surface of glass particles. The structuring of polyacrylic acid occurs due to the cross-linking of its linear molecules with polyvalent cations Ca2+ and Al3+.
The structuring mechanism is similar to the curing mechanism of carboxylate cements. Of all the polyvalent cations, Ca2+ has the greatest cross-linking activity. The presence of a sufficient amount of calcium ions in an aqueous solution of polyacrylic acid causes structuring within 15 s. In order to increase the working time (viability) to several minutes, calcium ions must be introduced into the solution gradually. This is achieved by the fact that calcium compounds are fused with glass and their leaching from glass particles with polyacrylic acid lasts for the first few minutes after mixing the powder with the liquid. After a sharp decrease in the concentration of calcium ions, the structuring of the polymer matrix mainly occurs due to aluminum ions. The interaction of polyacrylic acid with aluminum ions proceeds slowly (at 20 °C for several hours). The structuring of cement is completed after a few hours and Al3+ is responsible for it. In hardened cement, both calcium and aluminum salts are present in equal amounts.
Fluorine plays a special role in the structuring process. It is introduced into glass in the form of fluoride salts so that it can be more efficiently extracted with polyacrylic acid. Fluorine ion is a pH regulator. If fluoride ion is not extracted, the pH of the system rises rapidly and the cement is unusable. Fluorine and phosphate ions form insoluble salts, as well as complexes that play an important role in the transfer of ions and their interaction with polyacrylic acid.
Hardened cement is a heterogeneous material in which glass particles and phosphates are distributed in a combined organic-inorganic matrix. The strength and insolubility of the matrix is due to the formation of covalent bonds—Si—O—Si—O~ and cross-linking of linear polyacrylic acid molecules with polyvalent campons.
The powder and liquid are mixed on a plate. It is recommended to store the powder and mixing plate in the refrigerator, and the liquid at room temperature. The optimal powder/liquid ratio is 3:1. This ratio must be strictly maintained. Increasing the powder/liquid ratio leads to increased rigidity of the mixture, faster curing, increased compressive strength, hardness, and resistance to dissolution, but reduces working time (the viability of the molding compound).
By introducing various modifying additives into the cement liquid (polyacrylic acid solution), it is possible to improve individual characteristics of the material. By adding certain acids, the activity of which differs slightly from the activity of polyacrylic acid, the nature of the structuring of the system can be significantly improved. The introduction of acids promotes the extraction of metal ions from glass and their temporary binding in solution, which eliminates premature interaction of cations with polyvalent anions of polyacrylic acid. As a result, the curing speed is increased without reducing working time, which can even be increased. The best results were achieved with the introduction of tartaric acid. The concentration of polyacrylic acid significantly affects the properties of cement. By increasing the acid concentration, the powder/liquid ratio can be reduced, resulting in longer working times. As the concentration of polyacrylic acid increases, the solubility of cement decreases and the compressive and tensile strength increases linearly. Thus, it is desirable to use a solution that is as concentrated as possible. The limitation is the viscosity of the solution.
Indications for use. The hydrophilic nature of ionomer cements, high mechanical strength, adhesion to tooth tissues and basic dental materials provides them with a wide range of applications. recommends ASPA-IV for isolating coatings of occlusal surfaces of teeth, for filling fissures with caries and for restoring primary teeth, cavities of classes III and V, if they do not cover large areas of the enamel surface. ASPA-IV is also used to eliminate marginal defects of fillings and treat enamel erosion without cavity preparation, in particular cervical erosions. However, due to its lack of transparency and some fragility, ASPA-IV should not be used to restore class II and IV cavities or to restore large crown defects. When using ionomer cement as a sealant, it is applied in two stages: first, the material is of a liquid consistency, and then a thick one is applied to this layer. Due to their biocompatibility, glass ionomer cements are used without a backing.
When treating tooth tissue with a 50% citric acid solution, the permanence of adhesion of glass ionomer cements improves. It was found that after etching with a solution of citric acid, adhesion does not decrease for 3 months in water at a temperature of 37 °C. Fillings have high abrasive resistance, a low coefficient of thermal expansion (about 8•10-6 °C-1), and resist the effects of weak acids better than silicate cements. The cement contains leachable calcium fluoride CaF2. Glass ionomer cements are significantly superior to silicate cements in terms of adhesion and marginal seal density.
Great Encyclopedia of Oil and Gas
In addition to minerals, silicate cement contains a glassy mass, which is a eutectic melt from which minerals did not have time to separate due to the rapid cooling of the cement clinker. Glass consists mainly of uncrystalized ferrites, aluminates, dicalcium silicate, alkali compounds, and part of the magnesium oxide contained in the clinker. [31]
In addition to minerals, silicate cement contains a glassy mass, which is a eutectic melt from which minerals did not have time to separate due to the rapid cooling of the cement clinker. Glass consists mainly of non-crystallized ferrites, aluminates, dicalcium silicate, alkali compounds, and part of the magnesium oxide contained in the clinker. [32]
A porcelain spoon of silicate cement is poured into a test tube and distilled water is poured to half. Shake the contents of the tube vigorously for 2 - 3 minutes, then filter off the precipitate. [33]
Portland oil well cement is a type of silicate cement. This is a product consisting of a mixture of crushed materials of a given mineralized composition. The main part of Portland cement is clinker, which is obtained by firing a special mixture of limestone and clay (marl) until the components included in its composition are sintered (temperature approximately 1450 C). [34]
Portland cement cement is a type of silicate cement and consists of a mixture of crushed materials of a given mineralized composition. [35]
The maximum contraction value for most pure silicate cements is 8 - 9 ml per 100 g of cement. [36]
Properties
Silidont is a filling material belonging to the silicophosphate group. Available in powder form, consisting of 20% phosphate (visphate) and 80% silicate (silicin) cement. It is intended for filling carious cavities:
- in children on temporary teeth (premolars) with carious cavities of class I, II, V;
- in adults on permanent molars and premolars with carious cavities of class I, II, V.
For permanent teeth, before filling, it is necessary to make a spacer that will isolate the pulp.
Silidont is available in the form of a container with powder; liquid orthophosphoric (H3PO4) acid is added to it in a glass jar for mixing.
Silidont was previously called Erkodont mixed type and included zinc-phosphate and silicate powder. Currently, an improved Silidont-2, which is less toxic, is being produced for dental practice.
Silidont-2 is used to fill any carious cavities in all teeth, except those that do not extend to the labial and buccal surfaces. Available in powder form in a 50 g plastic jar, in 3 colors (from light yellow, yellow-gray to light yellow-gray) and 30 g. glass bottle with liquid for mixing it.
Silicophosphate cement Silidont is used for filling permanent, temporary chewing and anterior teeth and their contact surface. Medium and deep caries are filled with this product only when an insulating lining is created.
Indications, contraindications, side effects
Indications for use of Silidont:
- Filling of permanent and temporary teeth of molars and premolars in children.
- Filling in adult small and large molars, superficial type of caries, medium and deep, in the presence of isolation with other cement (containing zinc, phosphate).
- Fixation of fixed dentures.
Contraindications to the use of the composition are individual intolerance, allergy to one of the chemicals.
Side effects may occur due to non-compliance with the rules of mixing and applying the composition:
- Development of secondary caries.
- Filling falling out.
Composition, properties
The powder consists of 5 parts silicate and 4 parts phosphate cement, which are made.
The liquid is orthophosphoric acid, which has been partially neutralized with aluminum hydroxide (Al(OH)3) and zinc oxide (ZnO).
Properties of the drug:
- high mechanical strength;
- high chemical resistance;
- sets quickly;
- plastic;
- radiopacity;
- ensures the tightness of the filling, which reduces the risk of complications or relapse;
- bactericidal effect;
- high compatibility with biological tissues.
A significant advantage of the product is its low cost and the ability to purchase at any pharmacy chain.