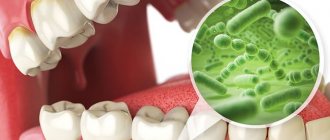
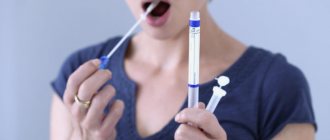
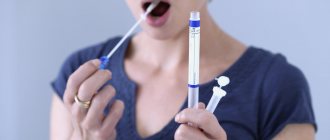
The main cause of dental caries is the activity of bacteria in the oral cavity. But there are also a number of additional factors that can contribute to the development of this disease. One of these factors is saliva. How the pH of human saliva affects dental health will be discussed in this article.
In this article
- Why does caries develop?
- What is the role of saliva in maintaining oral health?
- What happens when there is insufficient saliva production?
- How does saliva pH affect teeth?
- Prevention of salivary disorders and dental caries
- Conclusion
Why does caries develop?
Scientists are still studying the mechanisms of the development of caries - one of the most common diseases on the planet. They agree that caries is caused by microorganisms living in the oral cavity, in the presence of associated factors. These factors include carbohydrate nutrition, poor oral hygiene, and the qualitative and quantitative composition of saliva.
How does the carious process develop and why do cavities form in teeth? Contrary to popular belief, microbes do not chew holes in teeth; caries develops differently. Cariogenic bacteria (one of the species is Streptococcus mutans) form colonies on the surface of teeth, collecting in a biofilm - dental plaque. These bacteria “love” foods rich in carbohydrates and ferment the sugars contained in them. The products of carbohydrate processing by bacteria are organic acids. So they harm our teeth and contribute to the development of caries.
Acids produced by bacteria disrupt the normal structure of the enamel, promote its demineralization, penetrate into the deep tissues of the tooth and continue to destroy it from the inside. The more microbes in the mouth, the more carbohydrates they receive, the higher the acidity on the surface of the teeth and the more active the process of their destruction occurs.
The use of digestive enzymes in gastroenterological practice
P
Digestive enzymes are widely used for various gastroenterological pathologies.
Enzymes are actively used for various diseases of the stomach, small and large intestines, biliary tract and pancreas. Indications for the appointment of enzyme therapy
are disorders of the secretion of endogenous enzymes, disorders of absorption of nutrients and disorders of motility of the gastrointestinal tract. Currently, the global pharmaceutical industry produces a large number of enzyme preparations (Digestal, Creon, Mezim-Forte, etc.), which differ from each other both in the dose of digestive enzymes they contain and in various additives (see table). Digestive enzymes are available in various forms: tablets, powder or capsules containing enteric-coated microgranules. All enzyme preparations differ in their composition: containing pancreatin (pancreatic extract, usually of pork origin) or digestive enzymes, plant origin, extract of the gastric mucosa. The composition of the drug, in addition to enzymes, may include components of bile, gastric mucosa or adsorbents (simethicone or dimethicone).
Choice of drug
for the treatment of a patient with gastroenterological pathology should be based on the following indicators:
• absolute and relative content of enzymes in the drug (a high content of proteases is indicated for patients with decreased gastric secretion and a painful form of chronic pancreatitis; an increase in lipase activity is necessary for replacement therapy for pancreatic insufficiency);
• the presence of a shell that protects enzymes from digestion by gastric juice;
• the size of the tablet or granules filling the capsules (evacuation of the drug from the stomach simultaneously with food occurs if the size of its particles does not exceed 2 mm);
• the presence of bile acids in the composition of the drug (bile acids improve the digestion of lipids, increase the absorption of fatty acids and cholesterol, and cause increased pancreatic secretion). However, high levels of bile acids in the intestine during intensive enzyme therapy can cause hologenic diarrhea.
Digestive enzymes are indicated for patients with gastric secretion disorders
(hypo- and anacid chronic gastritis, conditions after gastrectomy). After surgical intervention on the stomach, malabsorption syndrome may develop, which is associated with a large number of different factors: a decrease in the production of hydrochloric acid and pepsin; violation of chyme mixing and mechanical processing; disruption of the fractional flow of chyme into the small intestine; acceleration of passage through the small intestine; decreased endogenous stimulation of pancreatic secretion; asynchronous flow of pancreatic juice, bile and chyme into the small intestine [17]. Treatment of post-gastroresection disorders requires complex therapy using antacids, agents that affect gastrointestinal motility, and digestive enzymes.
In hypoacid conditions accompanied by a decrease in the availability of nutrients, preparations containing bile are indicated. These drugs help increase the production of bile and pancreatic juice; they should be taken 1-3 tablets during or immediately after meals (without chewing) 3-4 times a day in courses of up to 2 months.
Preparations containing bile should be used with caution in patients with chronic hepatitis or cirrhosis of the liver, since bile acids enter the liver via the enterohepatic route, where they are metabolized, as well as in cholestatic diseases, peptic ulcers, Crohn's disease and ulcerative colitis.
Hypoacid gastritis is an indication for the prescription of drugs containing components of the gastric mucosa: pepsin and hydrochloric acid. These components provide mechanical and chemical processing of food, primarily proteins. Proteolysis occurs to the level of polypeptides, which are then broken down by pancreatic proteases and partially to amino acids. Pepsin stimulates pancreatic secretion and is therefore contraindicated in patients with chronic pancreatitis, especially in the presence of intraductal hypertension. Preparations containing pepsin are taken at the rate of 0.2–0.5 g of pepsin per meal, 2–3 times a day before or during meals. Recently, for the treatment of patients with a decrease in the acid-forming function of the stomach, drugs containing pure pancreatin, 1 t (4 times a day at the beginning of meals), have been successfully used.
For the treatment of hypomotor dyskinesia (hypokinesia) of the biliary tract
and disorders of fat solubilization, enzyme preparations containing bile acids are successfully used. Bile acids and salts increase the contractile function of the gallbladder and normalize the biochemical properties of bile.
Treatment of pancreatitis with severe pain
requires strict restriction of diet, prescription of antisecretory agents, antispasmodics and pure pancreatin preparations in high doses.
These remedies are the most universal for normalizing digestion in the gastrointestinal tract and can be used as part of complex therapy for all types of disorders. Enzyme products containing pure pancreatin contain proteases, amylase, and lipase. The drug Digestal
contains hemocellulase, which ensures the breakdown of cellulose. Modern research shows that digestive pancreatic enzymes in the traditional non-enteric form provide pain relief during exacerbation [20]. The entry of pancreatic enzymes (primarily trypsin) into the duodenum destroys the releasing peptides of secretin and cholecystokinin and causes a decrease in pancreatic secretion, ensuring functional rest of the organ (Fig. 1).
Rice. 1. Regulation of pancreatic secretion using releasing peptides (according to Li Y., Owyang C., 1996)
Much hope has been associated with digestive enzymes of plant and fungal origin, primarily due to the high acid resistance of plant and fungal lipases. However, under experimental conditions, bacterial lipase turned out to be 75 times less active than pork lipase, and therefore these drugs have not yet found use in clinical practice. Pancreatin does not affect gastrointestinal motility, bile secretion, or bile duct function.
Replacement therapy for exocrine pancreatic insufficiency
necessary for various diseases when atrophy of more than 90% of the organ parenchyma occurs [19] (Fig. 2), occurring most often in the late stage of chronic pancreatitis. Indications include steatorrhea (fat loss in feces more than 15 g/day, with a normal rate of up to 7 g/day), progressive weight loss, diarrhea, and dyspeptic symptoms. Treatment of exocrine pancreatic insufficiency still remains a challenge. Currently, therapy in several areas can be considered the most established: avoiding alcohol consumption, following a diet with frequent small amounts of food, enzyme replacement therapy, combating vitamin deficiency, analgesics (paracetamol, tramadol), psychotropic drugs.
Rice. 2. Etiology of exocrine pancreatic insufficiency
Enzyme therapy for the development of exocrine pancreatic insufficiency requires the use of capsules containing microgranules (microtablets) of pancreatin. In case of disturbances in the hydrolysis of nutrients, capsules are significantly more effective than regular-sized pancreatin tablets. The peculiarity of these drugs is that the enzymes they contain are released only in the alkaline environment of the small intestine and thus avoid destruction by gastric juice (Fig. 3), which significantly increases the effectiveness of replacement therapy for pancreatic steatorrhea. The stability of the drug in an acidic environment is a very important property of the drugs (the main components of enzyme preparations - lipase and trypsin quickly lose activity in an acidic environment: lipase at pH = 4, trypsin at pH = 3; before the drug enters the duodenum, up to 92% of the lipase can be destroyed ). This property significantly increases the effectiveness of replacement therapy and reduces or eliminates the need to prescribe drugs that block gastric secretion. Thus, when using a drug that has an enteric coating, fat absorption is on average 20% higher than when using a conventional drug in the same dose. However, in patients with chronic pancreatitis, the production of bicarbonates is significantly reduced, which leads to impaired alkalization in the duodenum and impaired activation of accepted enzymes. In this case, the effectiveness of the encapsulated enzymes may be significantly reduced.
Rice. 3. Effect of a microtablet drug with an enteric coating
The downside of enteric-coated drugs is that the enzymes do not have time to activate in the duodenum, the main site of production of pancreatic regulatory peptides. The low activity of proteases in the duodenum does not allow interrupting the stimulation of pancreatic secretion through a negative feedback mechanism and does not reduce the pressure in the ducts and parenchyma of the pancreas. High intrapancreatic pressure is considered the main mechanism for the development of intense pain in chronic pancreatitis, and therefore encapsulated enzymes are recommended only as replacement therapy, and for pain relief (especially with intraductal hypertension) - traditional pancreatin tablets or powder.
However, according to our own data, treatment of chronic pancreatitis using encapsulated pancreatin
led to a significant decrease in the intensity of abdominal pain in the examined patients (p=0.0063; Fig. 4). Moreover, the degree of pain reduction significantly depended on the degree of decrease in fecal elastase activity compared to the initial level (p = 0.0219). Thus, the analgesic effect of the therapy directly depended on the degree of suppression of the exocrine function of the pancreas in the patient. The exocrine function of the pancreas according to the elastase test (determination of fecal elastase using an immunoreactive method) significantly decreased compared to the background: 279.46±27.41 μg/g and 254.87±26.74 μg/g (n=52, p= 0.0152).
Rice.
4. Intensity of pain before treatment and while taking KPMES. The particle size of microgranulated preparations should not exceed 2 mm
, which ensures simultaneous evacuation of food and enzymes from the stomach. Further reduction in granule size does not lead to an increase in the efficiency of food digestion [13]. The administration of pancreatin capsules in a dose of 16–18 thousand units per meal to patients with chronic pancreatitis after surgical interventions on the pancreas (pancreaticoduodenectomy and drainage of the Wirsung's duct) for 2.5 years made it possible to reduce the loss of fat in feces from 33.6 g/day to 15.3 g/day [7]. There was a statistically significant increase in the body weight of patients and biochemical indicators of trophological status (serum iron, total iron-binding capacity of blood serum, total number of lymphocytes).
According to information obtained by Farkas G., Takacs T. et al. (1999), prescribing microgranulated pancreatin to patients after surgery on the pancreas at a dose of 25 thousand units. 3 times a day for 10 days did not lead to changes in pancreatic function compared to placebo [12]. Nevertheless, the therapy made it possible to effectively eliminate the symptoms of maldigestion, stabilize body weight (in the comparison group - weight loss of 3.5 kg over the same time) and increase the breakdown of carbohydrates by 35%.
Adequate therapy of exocrine pancreatic insufficiency syndrome requires the use of high doses of enzyme preparations. It is usually necessary to use enzymes in encapsulated form. The drug dosage regimen is as follows: 1–4 capsules of the enzyme preparation with main meals (at the beginning of a meal) and 1 capsule (tablet) with a small amount of food. The patient should avoid eating foods rich in fiber, as they reduce enzyme activity as in vitro
, and
in vivo
. The main component of the enzyme preparation, which determines the effectiveness of the treatment of digestive disorders, is lipase. At the same time, proteases and, above all, trypsin are the main inhibitors of lipase. Therefore, to relieve steatorrhea, one should not strive to significantly increase the proteolytic activity of chyme. Often, a high dose of enzymes entering the stomach does not provide the desired result: the main components of enzyme preparations, lipase and trypsin, quickly lose activity in an acidic environment. Therefore, the effectiveness of enzyme therapy can be increased by the simultaneous administration of antacid or antisecretory drugs, but it must be remembered that antacids containing calcium or magnesium weaken the effect of enzyme drugs.
Many open questions remain in assessing the effectiveness of enzyme replacement therapy, in particular, replacement therapy, and determining optimal doses. Thus, there are two groups of patients: patients in whom pancreatic secretion exceeds 10%, but nevertheless have steatorrhea; and those in which lipase secretion is practically absent, but normal digestion and absorption of fat is maintained. This may be due, respectively, to the different reserve secretory capacity of the pancreas and to the action of non-pancreatic lipases (produced by the mucous membrane of the tongue and stomach, mainly the upper part of the greater curvature). Researchers estimate that patients with exocrine pancreatic insufficiency may absorb more than 50% of dietary fat in the absence of detectable pancreatic lipase activity. It has been shown that in patients with severe pancreatitis with steatorrhea, when taking 100 g of fat with food, 20–50 g of fat can be absorbed without enzyme replacement therapy. According to Abrams CK, Hamosh M. et al. (1987) [5], in patients with pancreatic exocrine insufficiency, non-pancreatic lipases provide more than 90% of the total lipolytic activity at the level of the duodenojejunal junction, whereas in healthy people - 7%. This observation may, at least in part, explain why some patients do not require enzyme replacement therapy after total pancreatectomy.
However, in a double-blind crossover study, Neoptolemos JP, Ghaneh P. et al. (1999) in 37 patients with CP with exocrine insufficiency after extensive resection of the pancreas, there were no significant differences in stool frequency, fecal volume and daily loss of fat in feces while taking a standard or high dose of pancreatin. In this regard, the authors conclude that the main advantage of modern drugs with a high content of pancreatin in one capsule is convenience for the patient, which ensures more accurate adherence to the treatment regimen. In another study, when prescribing a capsulated drug to patients with chronic pancreatitis, no dependence of fecal fat content on the dose of the drug was found [15], which suggests the presence of a certain threshold of enzyme efficiency, upon reaching which a further increase in dose does not lead to a decrease in steatorrhea.
Treatment of patients with disorders of motor function and colon tone
, for example, with irritable bowel syndrome, in addition to antispasmodics, enveloping, psychotropic drugs, sometimes requires the use of digestive enzymes. The use of enzymes, which contain bile components, causes increased intestinal motility and helps resolve constipation in patients. Enzyme preparations that contain hemicellulase (Digestal) improve the digestion of plant foods and reduce bloating, which gives a good symptomatic effect.
Healthy individuals can take digestive enzymes to relieve dyspeptic symptoms after overeating
. Occasional intake of small doses of digestive enzymes (1-2 tablets) does not affect pancreatic function and is considered safe. In this situation, drugs with bile components have proven themselves to be the best.
The reasons for the ineffectiveness of replacement therapy may be associated with both inaccurate diagnosis of the disease and inadequate therapy. Sometimes a lower dose of the drug is prescribed to reduce the cost of treatment. Patients may incorrectly follow the prescribed treatment regimen: reduce the frequency of doses or take the enzyme at the wrong time (before or after meals). Enzyme preparations are ineffective for steatorrhea of extrapancreatic origin (celiac disease, giardiasis, etc.). The action of enzymes is impaired in intestinal motility disorders. Incorrect treatment regimen: prescription of enzymes that do not have an acid-protective shell without inhibitors of gastric secretion; the use of drugs that, due to the large size of the granules, do not enter the duodenum simultaneously with food.
Side effects of enzyme therapy are usually not severe. The most dangerous of them, the development of fibrosing colopathy, occurs in children with cystic fibrosis with long-term intake of very high doses of encapsulated enzymes - more than 50 thousand units of lipolytic activity per 1 kg of body weight per day. In addition, patients may experience pain in the oral cavity (usually when taking enzymes in powder form), skin irritation in the perianal area, and a feeling of discomfort in the abdomen. Long-term enzyme therapy in high doses can cause hyperuricemia, in some cases allergic reactions to pork protein occur (including in relatives of patients with exocrine pancreatic insufficiency and medical personnel). The formation of complexes with enzymes sometimes leads to impaired absorption of folic acid.
The list of references can be found on the website https://www.rmj.ru
Enzyme preparation –
Digestal (trade name)
(ICN Pharmaceuticals)
Literature:
1. Geller L. I., Pashko M. M., Obukhova G. G. Exocrine and endocrine pancreatic disorders in chronic pancreatitis. // Sov. Honey. – 1989. – No. 8. – pp. 4–7.
2. Yakovenko E.P. Enzyme preparations in clinical practice // Klin. pharm. and ter., 1998, No. 1, p. 17–20.
3. A primer of pancreatitis PGLankisch, M.Buchler, J.Mossner, S.Muller–Lissner // Springer, 1997.
4. Abrams CK, Hamosh M., Lee TC, Ansher AF, Collen MJ, Lewis JH, Benjamin SB, Hamosh P. Gastric lipase: localization in the human stomach. // Gastroenterol. – 1988. – Vol. 95. – P. 1460–1464.
5. Abrams CK, Hamosh M, Dutta SK, Hubbard VS, Hamosh P. Role of nonpancreatic lipolytic activity in exocrine pancreatic insufficiency. // Gastroenterol. – 1987. –Vol. 92. – P. 125–129.
6. Banks PA Acute and chronic pancreatitis. In: Sleisenger and Fordtran's gastrointestinal and liver disease: pathophysiology/diagnosis/management / Mark Feldman, Bruce F. Scharschmidt, Marvin H. Sleisenger–6th ed. WBSaunders company, 1998.
7. Braga M., Cristallo M., De Franchis R., Mangiagalli A., Agape D., Primignani M., Di Carlo V. Correction of malnutrition and maldigestion with enzyme supplementation in patients with surgical suppression of exocrine pancreatic function. // Surg. Gynecol. Obstet. – 1988. – Vol. 167, Dec. – No. 6. – P. 485–492.
8. Creutzfeldt W., Kern E., Kummerle F., Schumacher J. Die radikale Entfernung der Bauch–speicheldruse beim Menschen – Indikationen, Ergebnisse, Folgeerscheinungen. // In: Heilmeyer L, Schoen R, de Rudder B (eds) Ergebnisse der Inneren Medizin. – Springer, Berlin-Gottin-gen-Heidelberg. – 1961. – Vol. 16. – P. 79–124.
9. DiMagno EP Future aspects of enzyme replacement therapy. In Lankisch (Ed.) Pancreatic enzymes in health and disease, pp. 209–214, Springer–Verlag, Berlin, Heidelberg, 1991.
10. DiMagno EP Patterns of human exocrine pancreatic secretion and fate of human pancreatic enzymes during aboral transit. In Lankisch (Ed.) Pacreatic enzymes in health and disease, pp. 1–10, Springer–Verlag, Berlin, Heidelberg, 1991.
11. Diseases of the gut and pancreas. JJMisiewicz, REPounder, CWVenables eds., Blackwell scientific publication, 1994, vol. 1.
12. Farkas G., Takacs T., Baradnay G., Szasz Z. Effect of pancreatin replacement on pancreatic function in the postoperative period after pancreatic surgery. // Orv. Hetil. – 1999. – Dec 5. – vol. 140. – No. 49. – P. 2751–2754.
13. Halm U., Loser C., Lohr M., Katschinski M., Mossner J. A double-blind, randomized, multicentre, crossover study to prove equivalence of pancreatin minimicrospheres versus microspheres in exocrine pancreatic insufficiency. // Aliment. Pharmacol. Ther. – 1999 – Vol. 13. – No. 7. – P. 951–957.
14. Lankisch PG, Banks PA Pancreatitis. Springer-Verlag: Berlin, Heidelberg. – 1998. – P. 377.
15. Opekun AR Jr, Sutton FM Jr, Graham DY Lack of dose–response with Pancrease MT for the treatment of exocrine pancreatic insufficiency in adults. // Aliment. Pharmacol. Ther. – 1997, Oct. – Vol. 11. – No. 5. – P. 981–986.
16. Paris JC A Multicentre Double–Blind Placebo–Controlled Study of the Effect of a Pancreatic Enzyme Formulation (Panzytrat(r) 25,000) on Impaired Lipid In Adults with Chronic Pancreatitis //Drug Invest. 5(4):229–237, 1993.
17. Riley SA, Marsh MN Maldigestion and malabsorption. In: Sleisenger and Fordtran's gastrointestinal and liver disease: pathophysiology/diagnosis/management / Mark Feldman, Bruce F. Scharschmidt, Marvin H. Sleisenger–6th ed. WBSaunders company, 1998.
18. Roberts IM Enzyme therapy for malabsorption in exocrine pancreatic insufficiency. Pancreas 1989, #4, 496–503.
19. Sarles H., Pastor J., Pauli AM, Barthelemy M. Determination of pancreatic function. A statistical analysis conducted in normal subjects and in patients with proven chronic pancreatitis (duodenal intubation, glucose tolerance test, determination of fat content in the stools, sweat test). //.Gastroenterol. – 1963. – Vol. 99. – P. 279–300.
20. Stead RJ, Skypala I., Hodson ME Treatment of steatorrhoea in cystic fibrosis: a comparison of enteric–coated microspheres of pancreatin versus non–enteric–coated pancreatin and adjuvant cimetidine. // Aliment. Pharmacol. Ther. – 1988, Dec. – Vol. 2. – No. 6. – P. 471–482.
What is the role of saliva in maintaining oral health?
Saliva is the biological fluid of our body that is produced by the salivary glands. Constantly present in the oral cavity, saliva takes part in the digestive processes, nourishes tissues, provides natural cleaning of the oral cavity, and protects teeth and mucous membranes from bacterial exposure. All natural processes that occur with our teeth are carried out in interaction with this biofluid. Saliva ensures the normal functioning of all tissues and organs of the oral cavity; in particular, the important role of saliva in maintaining the mineral composition of tooth enamel has been proven.
In addition to water, saliva contains phosphates, calcium salts, fluorides, sodium compounds and other substances that help strengthen and maintain the health of tooth enamel. Essentially, salivary fluid is a solution supersaturated with calcium and phosphate salts. Due to this composition, saliva qualitatively mineralizes teeth. Medical research has proven that during teething, enamel matures primarily due to the active intake of calcium and phosphorus ions from saliva. It also maintains and, if necessary, restores the correct balance of minerals in already erupted teeth.
In addition to its mineralizing function, the protective role of saliva has been proven. It contains lysozyme and some other substances with bactericidal and bacteriostatic effects. Thanks to this, saliva neutralizes the action of pathogenic microorganisms and prevents the development of infections of the teeth and oral cavity. And finally, saliva performs a cleansing function, washing away food debris and germs.
Thus, normal quality and quantity of saliva is the most important condition for healthy teeth and oral cavity. Any violations of its quantitative or qualitative composition can provoke the development of caries, diseases of the mucous membranes and periodontium.
Thesiography - diagnostics of saliva. Saliva and its effect on oral health
What is the pH of saliva?
This is a pH value that helps determine the acidity of oral fluid.
Determination of the pH of saliva is carried out with special test paper strips soaked in acid and an acid-base indicator. A drop of saliva from a pipette is applied to such a strip; the saliva, to one degree or another, neutralizes the acidic impregnation of the strip, as evidenced by a change in the color of the indicator. Most often, the initial acidic pH level = 4.5 corresponds to a yellow-brown color, slightly acidic pH = 4.5-5.5 - green color, neutral pH - blue color. The closer the strip gets to the neutral state under the influence of buffer forces, the better.
The role of Ph in the development of caries
The reflux of acidic stomach contents through the esophagus into the oral cavity helps reduce the pH of saliva and plays an active role in the development of “local” pathological processes. After the hydrochloric acid has reached its destination, the Ph of the mixed saliva decreases to 7.
The secretion, which normally has alkaline properties, with an acidity level of 6–6.2 causes the destruction of tooth enamel, its demineralization, which subsequently results in the appearance of carious lesions. The gums become inflamed, swollen, red, and the amount of mucus on the mucous membrane increases noticeably. Oxygen-rich saliva prevents the proliferation of pathogenic microflora; with reduced acidity, these microorganisms “bloom.” Hunger, excitement, stress, pronouncing a long monologue, breathing through the mouth - these are all the factors that lead to a decrease in Ph. The same phenomenon is associated with natural age-related changes in the body.
The pH of mixed saliva allows us to judge the degree of demineralization of free dental tissues. This biological fluid constantly maintains a neutral acid-base reaction (average value - 7.2) of the oral environment due to the proteins and phosphates it contains.
According to the results of modern research, it is the prolonged exposure of acids to the hard tissues of teeth that provokes the appearance of carious lesions. When acidity decreases, saliva retains and binds calcium atoms, which leads to demineralization of teeth. Biological fluid prevents the dissolution of enamel and ensures the diffusion of calcium and phosphorus ions.
Saliva and its effect on oral health
It should be noted that the development of caries is associated not only with teeth and the activity of bacteria. The oral cavity represents
is a complex of organs and tissues in a moist environment (saliva). Saliva is a complex secretion. Oral fluid is commonly referred to as "mixed saliva." It primarily consists of the secretions of the major and minor salivary glands. In addition, it contains a number of components of non-salivary origin. These include: fluid of the gingival groove, serum components and blood cells, bacteria and their metabolic products, desquamated epithelium and cellular components, viruses and fungi, food debris and sputum secreted from the bronchi. Saliva is 99% water. The remaining 1% consists of large molecules of organic compounds such as proteins, glycoproteins and lipids, as well as small molecules of organic substances such as glucose and urea, electrolytes, mainly sodium, calcium, chloride and phosphates.
Mixed saliva is complete saliva without impurities that can be removed by centrifugation, or a mixture of pure saliva from all sources. Pure saliva is a liquid produced and secreted into the oral cavity by three pairs of large and many small glands. Every day, from 300 to 1500 ml of saliva is secreted into the human oral cavity. The production of saliva during the day is uneven: within 14 hours, without eating, approximately 300 ml of the so-called basic, unstimulated saliva is produced (salivation rate - 0.25-0.50 ml/min), within 2 hours, 200 ml is released against the background of food stimulated saliva (at a rate of 2.0 ml/min), and in the remaining time - 8 hours of night sleep - salivation practically stops (0.1 ml/min). At any given time, there is about 0.5 ml of saliva in the oral cavity. The prevalence and intensity of caries depend on the rate of salivation; these indicators are higher in children with a sharp decrease in salivation.
Saliva is well known to have protective properties against dental caries. The most direct evidence of this fact is the development of “blooming” caries following the cessation of the functioning of the salivary glands due to irradiation with high doses, due to tumors of the head and neck. Such caries is so destructive that within a few weeks it affects usually caries-resistant surfaces and causes complete destruction of the crowns of the teeth.
The main properties of saliva that provide protection against caries are the following:
- dilution and clearance of food sugars; • neutralization and buffering of acids in dental plaque; • providing ions for the remineralization process.
- lysozyme - can destroy the cell wall and dissolve bacteria; • lactoferrin - connects iron atoms to bacterial cells, which gives a bacteriostatic effect; • lactoperoxidase group - thiocyanin - H2 + O2 - the formation of oxygen springs gives a bactericidal effect, these sources accompany the reaction of many unsaturated fatty acids in the cell wall of the bacterium.
Saliva has a significant effect on the pH level of dental plaque, including preparing the food bolus for swallowing, as a result of which mechanical and chemical cleaning of the oral cavity occurs, and food debris that adheres to the smooth surface of the tooth is eliminated.
At the same time, they are partially purified enzymatically. Both of these processes are essential for the prevention of caries. Saliva protects the mucous membrane from damage, and also protects teeth from pathological abrasion by antagonist teeth. The enamel-saliva system can easily withstand a short-term “attack” of acid on the teeth.
Fluorine, phosphates and calcium found in saliva are the initiators of remineralization. The buffer system can neutralize acids to some level. In the moist environment of the oral cavity, acidic bacterial products cause constant pH fluctuations, which are neutralized by the buffer system. The buffer system is a protective element of saliva. Direct release of substances that inactivate bacteria:
The concentration of calcium and phosphates in saliva is very significant for the integrity of teeth.
Saliva is saturated with a solution of these ions. Inhibitory substances, similar to some bodies containing phosphates, block the spontaneous precipitation of the salt mixture on the surface of the enamel, which weakens the connection of microorganisms with the tooth surface. On the other hand, the solubility of mineral substances decreases. The reason for the restoration of superficial carious defects is not a single deposition of ions from saliva, but rather the recipitation of dissolved mineral substances removed from the deeper layers of the carious defect. The frequency of fluoride use plays an important role. Mineralizing potential of saliva
The mineralizing potential of oral fluid can be judged by the type of crystal-like formations in a drop of saliva placed on a glass slide. By the type of microcrystallization of saliva, one can judge the patient’s susceptibility to caries. Microcrystallization of saliva has individual characteristics and this may be associated with the general condition of the body, oral cavity, and nutrient load. The formation of microcrystals can characterize the remineralizing ability of saliva, and the intensity of caries is associated with the type of microcrystallization. In caries-resistant individuals, tree-shaped crystal-like formations are observed with a tendency for a drop of saliva to be located in the center.
In those susceptible to caries, this structure changes or disappears completely. It is believed that there is a close relationship between the structural and mineralizing properties of saliva.
There are three types:
Type I - a clear pattern of elongated crystalloprismatic structures fused together and occupying the entire surface of the drop. This type is inherent in the compensated form of caries.
Type II - in the center of the drop, individual dendritic crystalloprismatic structures of smaller sizes are visible than in type I. Characteristic of the subcompensated form of caries.
Type III - a large number of isometrically located crystalline structures of irregular shape are visible throughout the drop. This type of microcrystallization is characteristic of the decompensated form of caries.
At the same time, microcrystallization of saliva reflects the state of the body as a whole, so this parameter can be used for rapid diagnosis of some somatic diseases.
Conclusion
In the structure of dental morbidity, dental caries occupies one of the leading places, which in the absence of timely and correct diagnosis, which in addition to the main examination methods, additional ones should be used (such as test-measurement of saliva pH and thesiography). It is impossible to prescribe adequate treatment, which in turn will lead to the threat of the development of various odontogenic complications, the appearance of foci of chronic infection, functional disorders of the dental system, which have a negative impact on the patient’s health as a whole. Therefore, all efforts of specialists should be aimed at preventing dental caries using the latest technologies and materials available on the global dental market.
In our Jasmine family clinic, you can conduct a saliva pH test and tesiography (determining the mineralizing potential of saliva), where the doctor will tell you in detail about the health of your oral cavity and select an individual course of prevention.
What happens when there is insufficient saliva production?
According to statistics, every fifth adult is diagnosed with xerostomia, or dry mouth. This condition is caused by the fact that a person has reduced or completely absent saliva production. Since this biofluid performs a number of important functions, with xerostomia negative changes occur in the oral cavity:
- discomfort occurs;
- the level of hygiene of teeth and gums decreases;
- Due to a lack of saliva, the pH of the oral cavity decreases, microbes actively multiply in the mouth, and dental caries may begin to develop.
Saliva guards our teeth
Annotation. The article is devoted to saliva, which is a protector of tooth enamel. During experiments, the authors proved that saliva plays a huge role in maintaining the acid-base balance in the oral cavity. The authors offer recommendations that will help maintain a constant pH and preserve teeth from childhood. Key words: saliva, dental health, saliva acidity, pH, food acidity, saliva buffer capacity.
Tell me, why do we need saliva? To spit? In ancient times, humanity used saliva to protect itself by scaring away evil spirits; moistened sore spots and wounds; they spat on their hands before starting any hard work and even before fighting enemies, because they believed that saliva gave strength. In the modern world, thanks to new technologies, we have learned to detect various infections of the body through saliva, which helps to identify diseases at an early stage of development. Nature has created our body as a harmonious whole, in which there is nothing superfluous. This also applies to saliva, which plays a huge role in maintaining the normal condition of the organs and tissues of the oral cavity.
Research problem. If we didn’t have saliva, we would have dry mouth, which would cause multiple dental caries damage within 3-6 months. With a lack or absence of saliva, chewing and swallowing food becomes difficult.
Modern scientists have proven that one of the important properties of saliva is maintaining the required level of acidity in the oral cavity, since prolonged exposure to acids on tooth enamel leads to its destruction, and subsequently to the formation of caries. It turns out that saliva is an absolutely necessary component for health, because the health of the whole organism depends on healthy teeth.
Therefore, I decided to study how saliva maintains a healthy pH (acid-base balance) in the oral cavity and what level a person’s pH should be in order for their teeth to be healthy.
Hypothesis: is saliva really a protector of tooth enamel, restoring the acid-base balance in the oral cavity?
I set myself the following tasks:
1. Find out what saliva is and its properties.
2. Find out the importance of saliva in the development of dental caries.
3. Study what determines the acid-base balance of saliva.
4.Compare the pH of saliva from different people.
5.Measure the degree of acidity in food products and enter the acidity values into the table.
6. Determine the pH of saliva before and after meals. Find out how long it takes to restore balance in the oral cavity. Prove the presence of buffering properties of saliva.
7.Draw conclusions based on the research results.
To solve the problems, I used the following research methods: analysis of scientific literature; conducting an experiment to identify the real state of pH levels in people and food; visiting a dental clinic, etc.
From literature sources, I found out that saliva is a very important biological fluid for the body, which performs many functions in the body. The main ones are: digestive (wets the food bolus, thereby preparing it for swallowing and digestion), excretory (removes harmful products at the time of spitting saliva), mineralization (supplies vitamins and microelements), buffer (neutralizes acids produced by bacteria and the acidic contents of the stomach) , moisturizing (prevents the mucous membrane from drying out) and others. From the article “Interesting facts about saliva” [1], I learned that a healthy person constantly produces saliva; on average, 1-2.5 liters of saliva are released per day. Salivation increases under the influence of olfactory and gustatory stimuli, and decreases under stress, fear and practically stops during sleep, which is why we experience bad breath in the morning. This occurs because the oral cavity dries out, and saliva is less able to fight the microorganisms living there. Therefore, it is very important to brush your teeth at night!
After visiting a dentist, I found out that regular saliva can reduce the risk of caries, because it performs a protective function for the oral cavity: it maintains a healthy pH (acid-base balance); delivers beneficial phosphates and calcium to teeth; washes away plaque from easily accessible places; does not allow stone to form on the surface of the tooth; destroys the cells of “bad” bacteria; helps “good” bacteria by supplying them with nitrates (salts of nitric acid) for respiration, etc. Regular visits to the dentist and proper oral hygiene allow you to have healthy saliva, which is very necessary for the human body. Anna Andreevna said that through saliva you can detect not only diseases in the oral cavity, but also the whole body.
To do this, saliva is analyzed using the “immersed slide” method, introduced into dentistry in 1976. The principle of this technique is very simple. Saliva is collected on a plastic “slide”, after some time the results are compared and diseases are identified.
Saliva analysis is a very unusual and even unconventional diagnostic method. But despite this, saliva testing is gaining popularity. Saliva analysis is a modern diagnostic method that is used to determine various infections in the body. With the help of saliva analysis, you can conduct a DNA test to determine paternity and learn a lot about the metabolism in the body - how to lose weight.
Today, in many pharmacies you can buy a test and carry out the analysis at home. Using this analysis, it is possible to identify dysbiosis of the oral cavity, which is one of the symptoms of diseases of the gastrointestinal tract. Saliva analysis is also carried out to determine drugs and alcohol in the human body.
Very often, dentists use saliva analysis in their practice, which allows them to predict the possibility of caries development.
I believe that an indicator of human health is healthy, strong teeth. Teeth with strong enamel are less susceptible to aggressive substances. Some people's saliva is more alkaline, so the acid produced by bacteria is neutralized and tooth decay does not develop. From the article “Protective properties of saliva”[2], I found out that the secreted acids dissolve the surface of the tooth, hence the tooth enamel becomes weak. Currently, the occurrence of dental caries is associated with changes in pH in the oral cavity. Having studied the article “What is pH and acid-base balance” [3], I learned that the acidity of each person’s saliva is individual. Typically, the acidity of mixed human saliva is 6.8 to 7.4 pH. In children, on average, the acidity of mixed saliva is 7.32 (higher), and in adults it is 6.40 pH (lower). This is explained by the fact that over the years the composition of food, the condition of the oral cavity, oral care, and the speed and volume of salivation change. Therefore, it is very important to maintain a constant acidity level (pH), and not allow it to deviate noticeably, primarily in the acidic direction, which is fraught with serious disturbances in the function and composition of tooth enamel.
After studying the literature, I made recommendations that will help maintain a constant pH and preserve teeth from childhood:
1. Do not abuse sweet and carbonated drinks, which lead to a decrease in pH value, and subsequently to the formation of dental plaque.
2. Eat vegetables, fruits, lean meat, milk, as after eating them
no plaque remains.
3. After eating food, rinse your mouth with water or tooth elixir, nibble on an apple or carrot.
4. Drink plenty of fluids when you are sick and taking medications, as salivation decreases during this period.
5. Don’t smoke, because this reduces salivation, which means the pH level decreases.
6. Follow the rules of dental hygiene, don’t forget to brush your teeth!
7. Visit the dentist more often for preventative purposes.
In order to prove that saliva has a buffer capacity, that is, it is capable of neutralizing acids and alkalis, and being a protective mechanism when, first of all, acidic foods act on tooth enamel, I decided to conduct an experiment using a pH meter and indicator paper to identifying the degree of acidity in food and in the oral cavity.
Since one of the factors in the preservation of teeth is nutrition, I conducted an experiment to determine the degree of acidity in frequently consumed foods and compiled a table where I indicated the acidity values (Appendix, Table 1). If I saw a pH value below 7 - I have an acidic environment, a value equal to 7 - a neutral environment, above 7 - an alkaline environment. The result of the experiment showed that the products have their own acidity. This means that by eating foods containing high acidity, we can harm tooth enamel.
My next experiment was to compare the pH levels of people of different ages. A study with indicator paper showed that there is a difference in the color of the stripes from yellow to deep green, this indicates a different pH value: approximately from 5 to 8. This observation allows you to warn about a deviation in the pH level, therefore, for a more accurate measurement of the pH value, I use pH meter with an accuracy of 0.1 pH. According to the results of my research (Appendix, Table 2), I saw that the average pH of a child’s saliva (with healthy teeth) is 7.46; and the average pH of adult saliva is 6.56. A child was also identified with “bad” teeth affected by caries - pH -5.5. For people with a lower pH reading, I recommended visiting a dentist and then following my recommendations. The results of experiments carried out by two different methods showed approximately equal values, that in people of different ages the degree of acidity of saliva is not the same: in children the pH value is above 7, that is, saliva is slightly alkaline in nature, and this means less likelihood of developing caries.
In order to show how much saliva can neutralize acids, i.e. perform a protective function in the oral cavity, I decided to determine the pH of saliva before and after eating the foods studied earlier. Next, in order to track how long it takes for the acid-base balance in the oral cavity to be restored, I measured the pH of saliva 10 minutes after eating (Appendix, Table 3). After eating, the pH value is: lower if you consumed a highly acidic product; normal and higher if you consumed a low-acid product or slightly alkaline products.
After 10 minutes, I again measured the pH level again.
According to the results of the experiment, we saw that the pH value of saliva became higher 10 minutes after consuming the product, which means that saliva neutralized the acidic environment, thereby preventing a destructive effect on tooth enamel.
I have proven that saliva actually protects tooth enamel by maintaining the acid-base balance in the mouth.
Conclusions: 1. Having studied the properties of saliva, I concluded that it moistens the oral cavity, promoting articulation, provides the perception of taste, lubricates and glues chewed food, promoting swallowing. 2. I found out that one of the most important properties of saliva is maintaining the required level of acidity in the oral cavity. 3. I found out that food affects the condition of teeth: consuming foods with a pH level below 7 (acidic) for a long time increases the acidity of saliva, which increases the risk of caries. 4. After analyzing the experiments and information, I came to the conclusion that saliva plays a huge role in maintaining the acid-base balance. Neutralizing the acidic environment, alkalizing it, saliva performs a protective function for tooth enamel.
From the literature sources I studied, I found out that our body is able to properly absorb and accumulate minerals and nutrients only with the proper level of acid-base balance. We have the power to help our body receive, rather than lose, nutrients. To do this, you need to maintain the correct pH balance. As a result of my research, I found out that saliva will help maintain the desired pH value in the oral cavity and prevent it from noticeably deviating in one direction or another. Saliva cleanses the oral cavity, has a bactericidal effect, protects teeth from damage, performs numerous functions and thereby helps our body.
The quality of saliva and the presence of beneficial properties in it directly depends on the general condition of the oral cavity, as well as on the health of teeth and gums in particular. Therefore, regular visits to the dentist and compliance with the rules of oral hygiene, as well as the “correct” composition and nature of food and water consumed, allow you to have healthy saliva, which, as it turns out, is very necessary for the human body.
Links to sources
- https://i-fakt.ru/interesnye-fakty-o-slyune.html
- https://us-in.net/ph-balance.php
- https://www.stomfak.ru/terapevticheskaya-stomatologiya/zaschitnye-svojstva-slyuny.html
- https://vitaportal.ru/stomatologiya/analiz-slyuny-pokazyvaet-risk-razvitiya-kariesa.html
- https://simptom.net/articles/esli-vam-ne-naplevat-slyuna-i-organizm/
- https://zenslim.ru/content/
- https://ru.centrmed.com/news/detail.php
- https://www.vashaibolit.ru/2328-zdorovye-zuby-zdorovyy-chelovek.html6.
- https://ru.wikipedia.org/wiki/
- "Environmental Chemistry", 2006
Table 1. Product acidity values
Table 2. Results of pH levels in people of different ages
How does saliva pH affect teeth?
One of the protective elements of saliva is the so-called buffer system. Its task is to neutralize the negative effects of acids entering the oral cavity. Buffer systems maintain a normal pH level in the oral cavity, providing protection against acid-base influences. It is the buffer systems of saliva that influence the restoration of the pH of the oral cavity within minutes after eating.
If the pH of saliva is disturbed, buffer systems do not cope with their task and cannot neutralize acids. This leads to the fact that tooth enamel quickly loses mineral substances, caries develops, as well as non-carious lesions of dental tissues.
Dietary habits and poor oral hygiene can affect changes in the pH level of saliva, and therefore play a role in the development of caries. Some diseases and conditions also affect the process of salivation. For example, with Sjogren's syndrome and diabetes, saliva production decreases, dry mouth and related problems occur. In cases of dysfunction of the thyroid gland, rheumatoid arthritis, and some mental illnesses, saliva does not perform its mineralizing function, that is, it does not provide tooth enamel with a sufficient amount of calcium and phosphorus ions. This explains the fact why, with concomitant diseases, caries in patients is much more common and can develop at a faster pace.
Research also shows that the normal pH level of saliva is different for children and adults. In children, the enamel begins to lose minerals at higher pH levels, and the acid-base balance in the oral cavity is restored more slowly due to the smaller volume of saliva compared to adults. Therefore, caries in childhood develops much faster.
Why is saliva needed?
Human saliva is a complex biological fluid consisting of 98.5% water. The source of saliva is the major and minor salivary glands. A healthy adult produces about 0.5-2.0 liters of saliva per day. Saliva performs many functions.
!!! The constant flow of saliva plays the role of a cleansing mechanism, helps remove food debris from the teeth, prevents the attachment of microorganisms, and also moisturizes the mucous membranes of the oral cavity, preventing them from drying out and becoming inflamed.
!!! The released saliva weakens the effects of aggressive substances, high and low temperatures, food and drinks on the mucous membrane and teeth.
!!! The enveloping properties of saliva are provided by a complex protein - mucin. It forms a viscous layer of mucus that binds the pathogen, preventing germs from entering. Possessing viscosity, mucin glues and moisturizes food particles that irritate the root of the tongue. As a result of swallowing, the elastic food bolus easily enters the esophagus and then into the stomach.
!!! Saliva has pronounced bacteriostatic and bactericidal properties due to the enzyme Lysozyme contained in it. It destroys the cell walls of many pathogenic bacteria, preventing their growth and reproduction. Lysozyme does not remain permanently in the oral cavity. With frequent spitting of saliva, it is lost.
!!! The blood-clotting function of saliva is determined by the presence in the oral fluid of plasma elements and blood clotting factors - thromboplastin (a product of the destruction of blood platelets - platelets) and prothrombin.
!!! Saliva maintains an optimal acid-base balance in the mouth due to its ability to neutralize acids, thereby reducing the aggressive effects of acidic foods, drinks, and products of microorganisms on the oral organs.
The acidity of each person's saliva is individual; in some people, the saliva is more alkaline, so caries almost does not develop.
!!! Saliva is the first digestive juice. The primary biochemical processing of the food bolus occurs in the oral cavity under the influence of saliva. It contains the enzymes Amylase and Maltase, which alternately act on starch (polysaccharide) and break it down into glucose.
!!! Saliva nourishes the enamel of teeth - those reserves of minerals that teeth lose due to changes in pH levels in the mouth are again supplied to them from saliva. Normally, both of these processes balance each other. But if the balance is disturbed (the leaching of minerals begins to occur faster than their replenishment), caries develops.
Impaired salivation is directly related to the destruction of enamel and frequent cases of caries. As a result of a decrease in the amount of saliva, plaque formation on the teeth increases, and dental plaque is formed. It leads to demineralization of enamel and the development of caries, and also contributes to the appearance of inflammatory periodontal diseases.
Prevention of salivary disorders and dental caries
Saliva and tooth decay are closely related, and it is important to have enough saliva in your mouth to protect your teeth. The following preventive measures will help prevent disruption of salivary processes and minimize the risks of dental caries:
- Drink enough water and other fluids to keep your body hydrated.
- Maintain good oral hygiene: brush your teeth twice a day, thoroughly removing plaque. Pay attention to the interdental spaces, gums and tongue. It is recommended to additionally use irrigators. These devices clean hard-to-reach areas of the oral cavity using a water jet. In this way, you can not only remove contaminants, but also moisturize the mucous membranes in case of increased dryness.
- Give up bad habits; smoking and drinking alcohol reduce saliva production, lead to dry mouth, and therefore increase the risk of dental caries.
- For xerostomia, on the recommendation of a doctor, you can use special rinsing solutions that increase the hydration of the mucous membranes.
- If necessary, the doctor can prescribe drugs that imitate the natural component of saliva.
- Sometimes salivation decreases due to taking some medications. In this case, it is recommended (after consultation with a doctor) to discontinue them or limit their use.
- It is equally important to normalize nutrition by limiting the amount of carbohydrates and sweet foods, which are a favorable environment for the development of pathogenic microorganisms, greatly disturb the pH of the oral cavity after eating, and are one of the most important factors in the development of caries.
- And, of course, to prevent any dental diseases, it is important to visit a doctor twice a year. Along with a traditional dental examination, modern clinics offer salivary pH tests and thesiography. This is a diagnostic method that will help determine the mineralizing potential of saliva.
the acid-base balance is normal or (acid-base indicator) of any necessary liquid and mixtures of liquids, such as the environment of the human body (urine, saliva, feces, semen, vaginal environment , breast milk) and external media (solutions, water, drinks, etc.).
Why you need to know this
The fact is that, starting from the 90s of the 20th century, speculation about the compliance of any product, according to the pH indicator, with the human body has become a very common phenomenon: we are told about this from soap advertising to advice on which muesli to choose for breakfast.
Although for most people, to be honest, this indicator does not mean anything, since most do not remember either the school course in chemistry or biology, and modern schoolchildren are simply not told about this. So manufacturers take advantage of general illiteracy, introducing into consciousness the usefulness of their products through the use of scientific information.
But is it really that simple?
Not really. The fact is that all human environments: urine, saliva, feces, semen, vaginal lubrication, breast milk, etc. - have different acidity-alkalinity indicators.
Moreover, if we take the entire gastrointestinal tract, which begins in the oral cavity and ends in the rectum, it has different pH in its sections. This means that the equation of the entire organism to a single pH value can lead to illness and death ! This is why pH is also called acid-base EQUILIBRIUM.
What is pH
The ratio of acid and alkali in any solution is called acid-base balance (ABC), although physiologists believe that it is more correct to call this ratio the acid-base state.
KSHR is characterized by a special pH indicator (power Hidrogen - “hydrogen power”), which shows the number of hydrogen atoms in a given solution. At a pH of 7.0 they speak of a neutral environment. The lower the pH level, the more acidic the environment (from 6.9 to 0). An alkaline environment has a high pH level (from 7.1 to 14.0).
The human body is 80% water , so water is one of its most important components. The human body has a certain acid-base ratio, characterized by pH (hydrogen) value.
The pH value depends on the ratio between positively charged ions (forming an acidic environment) and negatively charged ions (forming an alkaline environment).
The human body constantly strives to balance this ratio, maintaining a strictly defined pH level. When the balance is disturbed, many serious diseases can occur.
pH, or indicator of acid-base balance
It is a measure of the relative concentration of hydrogen (H+) and hydroxyl (OH-) ions in a liquid system and is expressed on a scale from 0 (complete saturation with hydrogen ions H+) to 14 (complete saturation with hydroxyl ions OH-), distilled water is considered neutral with pH 7.0.
An increase in the concentration of positive hydrogen ions (H+) in any body fluid causes a shift in pH values towards zero and is called an acid shift. An increase in the concentration of hydroxyl ions OH causes a shift in pH values towards 14 and is called an alkaline shift.
A very long time ago, substances were discovered in chemistry that have the ability to change their color in the presence of acids and alkalis. These substances are called indicators and are used to determine the reaction medium.
The environment can be acidic, alkaline and neutral. It is thanks to them that in modern medicine it is possible to saturate filter paper with litmus. Litmus is a coloring substance extracted from certain types of lichen, actually a weak acid. Its composition is complex.
How to use indicator paper:
It is necessary to dip a strip of paper into the required solution for two to three seconds. Compare with the supplied color chart and calculate the values.
pH STANDARDS
Solutions and liquids with respect to their acidity are considered:
- neutral at pH = 7
- acidic at pH < 7 alkaline at pH > 7
You can measure the acidity of any liquid medium. It is important for us to know and understand the state of our body. But remember that healthy acidity differs in different environments of the body.
Acid-base balance. Acidity of urine
If the urine pH level fluctuates between 6.0 - 6.4 in the mornings and 6.4 - 7.0 in the evenings, then the body is functioning normally. The most optimal level: slightly sour, in the range of 6.4 - 6.5. A urine pH value below 5.0 indicates that it is strongly acidified, and above 7.5 indicates that it is strongly alkaline.
The reaction of urine determines the possibility of stone formation : urate - in an acidic environment, oxalate - in a neutral-acidic environment, phosphate - in a more alkaline environment.
For example, uric acid stones virtually never occur when the urine pH is greater than 5.5, and phosphate stones never form unless the urine is alkaline. The best time to determine the pH level is 1 hour before or 2 hours after a meal .
Check the pH level twice a week 2-3 times a day.
Using indicator litmus paper pH test, you can easily, quickly and accurately monitor the response of urine to changes in the type of diet, the use of medications or dietary supplements.
Positive pH dynamics can serve as a criterion for the correctness of the chosen diet or treatment. The acidity of urine varies greatly depending on the food taken, for example, eating plant foods increases the alkaline reaction of urine. The acidity of urine increases if a person’s diet is dominated by meat foods rich in proteins.
Heavy physical work increases the acidity of urine.
Increased acidity of urine is observed with increased acidity of the stomach. Reduced acidity of gastric juice does not affect the acidity of urine.
The acidity of urine changes in many diseases or conditions of the body, so determining its acidity is an important diagnostic factor.
The “Star of Organs” cleansing system will help bring the body into a healthy state.
Acid-base balance. Saliva acidity
The acidity of saliva depends on the rate of salivation. Typically, the acidity of mixed human saliva is 6.8–7.4 pH, but with high salivation rates it reaches 7.8 pH. The acidity of the saliva of the parotid glands is 5.81 pH, of the submandibular glands - 6.39 pH. In children, the average acidity of mixed saliva is 7.32 pH.
Optimal measurement from 10 to 12 hours. It is better to measure it on an empty stomach, two hours before or two hours after a meal. Salivation decreases in the evening and at night.
To increase salivation, in order to increase the pH of saliva, it is good if there is a piece of lemon on the plate; it even with visual perception increases salivation.
Food should look appetizing, served on beautiful dishes, appetizingly decorated with herbs and/or vegetables, it should, as they say, please the eye! Not only the saliva flows, but also the juices in the body, preparing for the process of digesting food. This is the mental phase of digestive secretion.
Saliva, which normally has alkaline properties, at low pH, especially at values of 6.2–6.0, leads to focal demineralization of tooth enamel with the appearance of erosions of hard dental tissues and the formation of cavities in them - caries. The amount of mucus on the mucous membrane increases, the gums become swollen and inflamed.
When the acidity in the oral cavity decreases, the acidity of dental plaque decreases, which causes the development of caries.
Bacteria in the mouth thrive in the absence of air. Saliva, rich in oxygen, actively prevents their reproduction. Bad breath occurs when the flow of saliva slows down, such as during sleep.
Excitement, hunger, pronouncing a long monologue, breathing through the mouth (for example, with a runny nose), stress - dry out the oral cavity, leading to a decrease in the pH of saliva. A decrease in saliva flow inevitably occurs with age.
You can set the desired pH of water for rinsing or ingestion using litmus indicator paper. There cannot be recipes with the required proportions, because... Each region has its own water, with its own pH. Therefore, it is necessary to have indicator paper on hand.
Acid-base balance. Vaginal acidity
The normal acidity of a woman's vagina ranges from 3.8 to 4.4 pH and averages 4.0 to 4.2 pH.
Vaginal acidity in various diseases:
- cytolytic vaginosis: acidity less than 4.0 pH
- normal microflora: acidity from 4.0 to 4.5 pH
- candidal vaginitis: acidity from 4.0 to 4.5 pH
- Trichomonas colpitis: acidity from 5.0 to 6.0 pH
- bacterial vaginosis: acidity greater than 4.5 pH
- atrophic vaginitis: acidity greater than 6.0 pH
- aerobic vaginitis: acidity greater than 6.5 pH
Lactobacilli (lactobacillus) and, to a lesser extent, other representatives of normal microflora are responsible for maintaining an acidic environment and suppressing the growth of opportunistic microorganisms in the vagina. In the treatment of many gynecological diseases, restoration of the lactobacilli population and normal acidity comes to the fore.
Read also: How to properly maintain feminine hygiene.
Acid-base balance. Sperm acidity
The normal acidity level of sperm is between 7.2 and 8.0 pH. Deviations from these values are not in themselves considered pathology. At the same time, in combination with other deviations, it may indicate the presence of a disease.
An increase in the pH level of sperm occurs during an infectious process. A sharply alkaline reaction of sperm (acidity approximately 9.0–10.0 pH) indicates prostate pathology.
When the excretory ducts of both seminal vesicles are blocked, an acidic reaction of the sperm is observed (acidity 6.0–6.8 pH).
The fertilizing ability of such sperm is reduced. In an acidic environment, sperm lose motility and die. If the acidity of the seminal fluid becomes less than 6.0 pH, the sperm completely lose their motility and die.
Read also: How to maintain and restore men's health.
The acidity of tears is normal - from 7.3 to 7.5 pH.
The acidity of feces is normally from 6.0 to 8.0 pH.
The acidity of human breast milk is 6.9-7.5 pH
Acid-base balance. Blood acidity
(here you will need special strips with a narrower and more detailed measurement range) The acidity of human arterial blood plasma ranges from 7.37 to 7.43 pH, averaging 7.4 pH.
The acid-base balance in human blood is one of the most stable parameters, maintaining acidic and alkaline components in a certain balance within very narrow limits.
Even a small shift from these limits can lead to severe pathology. When shifting to the acidic side, a condition called acidosis occurs, and to the alkaline side, alkolosis occurs. A change in blood acidity above 7.8 pH or below 6.8 pH is incompatible with life.
The acidity of red blood cells is 7.28–7.29 pH.
Normal blood revitalizes lymph cells that can destroy tumor cells. There are many lymphatic cells (eg NK cells, LAK cells) in the human body.
Their uniqueness lies in the fact that they are able to distinguish normal cells from diseased and damaged ones, and destroy the latter. This is the function of the human body's immunity . The greatest activity of lymphatic cells in destroying diseased cells occurs at pH 7.4.
However, usually around the affected cells, there is a more acidic environment, which interferes with the activity of lymphocytes, which work better at a slightly alkaline pH. By consuming foods that have an alkalizing effect, you can adjust the pH balance within 0.5 units, creating a favorable environment for the action of lymphocytes and the destruction of affected or abnormally constructed cells.
Cancerous tissue has increased acidity, unlike normal tissue, and the body protects it with a fibrous membrane, which has an alkaline pH. If you continue to follow the acidic diet, the membrane dissolves and the cancer cells are released.
Once a week, when the body is acidified, it is advisable to arrange treatment days for yourself , eating only vegetables (1.5 kg of vegetables, divided throughout the day), boiled and sometimes raw in the summer, only heat-treated in the autumn-winter) and Be sure to have clean hot water.
In addition, remember that a violation of the pH of the body’s environment leads not only to the development of diseases, slagging of the body, but also to hormonal changes, which affects a person’s metabolism, his mood, and character.
A person’s mood is also important for maintaining a normal pH level in the body; a good, cheerful mood normalizes the acid-base balance. Laugh more!
HEALTH OF BODY, SOUL AND SPIRIT
Enter your email and receive the most interesting and useful materials about the practices of preserving and restoring health that our ancestors used!
GET USEFUL MATERIALS
By clicking on the button, you confirm your consent to the processing of personal data in accordance with the Privacy Policy.
Thank you!
We will contact you soon.
P.S. Do you monitor the acid-base balance of the body? Is your acid-base balance disturbed or is it normal?
Share in the comments!
- 76shares
- Vk43
- Ok29
- Fb4
- WhA
- Vib
- Tel
- Sk