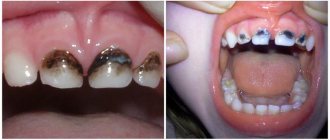
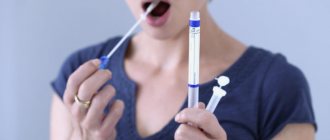
Galvanosis
The oral cavity is a complex biological environment, in the soft tissue structures and mucous membranes of which a large number of electrochemical reactions occur under the influence of saliva. One of them is the occurrence of galvanic microcurrents - weak impulses of electricity. They are formed when salivary fluid comes into contact with metal orthopedic devices in the mouth. This process is the norm, however, with an increase in the strength of the impulses, pathologies can form, primarily galvanosis. Previously, this was called intolerance to metal alloys and their inclusions in the oral cavity.
Specialists from the branches of family dentistry West Dental in Vsevolozhsk microdistrict Yuzhny and Yanino-1 will first of all take a detailed allergy history at a consultation appointment and help you choose orthopedic and surgical structures for your convenience. Dentists, orthopedists-surgeons-implantologists Epimakhov Konstantin Vasilyevich and Berkovich Sergey Andreevich will make the installation and selection of the necessary structures comfortable and most suitable for you.
What is galvanic syndrome
Galvanic syndrome is encountered by people who have metal prostheses made of dissimilar metals installed on their teeth (fillings, crowns, inlays, implants). Oxides of certain metals, which have different electrochemical activity and come into contact with a liquid electrolyte medium (in this case, saliva), provoke an increase in galvanic currents in the oral fluid. In a simplified version, electrical impulses appear between prostheses made of different metals due to their chemical properties. This process is called galvanism.
And the pathology called “galvanosis” becomes a consequence of galvanism. That is, galvanosis is already a diagnosis. With galvanosis, the patient notices pathological symptoms - for example, a metallic taste in the mouth (more on this below), and if left untreated, very serious consequences for the entire body are possible. By the way, there are quite a lot of patients who, to one degree or another, have encountered galvanic syndrome in the mouth - from 15% to 35% of all cases of prosthetics.
Complex on 4 OSSTEM implants with delayed loading - from RUB 170,000.
Complex implantation Osstem (South Korea) with delayed loading after 4-6 months.
Guarantee for the doctor’s work - unlimited Call now or order a call
Opening hours: 24 hours a day - seven days a week
Interestingly, the phenomenon of galvanism in a living organism was first discovered, but not studied in detail, by the Italian scientist Luigi Galvani, after whom the process was named. Although in fact, the true meaning of galvanic currents (including in the body) was revealed by Galvani’s student Alessandro Volta. Moreover, Volta went further than his teacher and made many discoveries in the field of electricity, and also developed the first battery, the operating principle of which was based on chemical processes.
Definition of pathology
Galvanosis (ICD code K13.7) is a pathology in dental practice that occurs due to the strong influence of metal currents on the soft tissue structures of the oral cavity. Such currents are formed when metal structures of different potentials come into contact with each other and with salivary fluid.
Characteristic signs of the occurrence of galvanism are:
- metallic taste in the mouth;
- dry mucous membranes;
- decreased taste sensitivity;
- burning of the papillae of the tongue;
- frequent migraines;
- increased irritability.
The first symptoms become noticeable within a couple of months after the installation of prostheses containing steel, clasps made of chromium-cobalt, and replacement of the old bridge-like console with a new one made of a gold alloy.
Signs
The syndrome does not develop immediately; the patient does not feel discomfort for some time. But the complexity of the situation is that if it worsens, there is a high risk of developing complications that negatively affect health. Signs of galvanism that you should pay attention to are:
- fatigue, lethargy, without reason;
- sleep disturbances, difficulty falling asleep, frequent awakenings at night;
- disability;
- sharp headaches;
- colds;
- sudden mood changes, unmotivated irritability.
Additionally, symptoms may develop that indicate the need to see a doctor for examination:
- a metallic taste appears that does not disappear even when eating;
- a sour taste appears, the sense of taste of foods changes;
- itching, numbness of soft tissues, tingling, sharp burning appear;
- a feeling of current appears on the mucous membrane;
- mucous membranes, soft tissues become sensitive to touch;
- change in the amount of saliva produced.
Causes
Modern orthopedic specialists identify a number of fundamental reasons for the formation of galvanosis after prosthetics with devices containing metals. It is very important to understand the causes of galvanosis in order to correctly perform a differential diagnosis in the clinic and draw up the necessary treatment plan.
- Galvanic cell. Reasons for its formation:
- difference in potentials of the metals that make up the alloy of the prosthesis;
- different composition and structure of metal combinations;
- non-compliance with the rules for casting material;
- low-quality alloy;
- error in assigning dissimilar metals.
- Toxic effects of heavy metals.
- Individual patient health characteristics and concomitant diseases:
- intolerance to alloy components of the prosthetic structure;
- characteristics of salivary fluid;
- chronic lesions - caries, periodontitis;
- pathologies of the gastrointestinal tract;
- poor quality oral hygiene.
Prevention of galvanic syndrome
In order to prevent galvanosis, you should carefully choose a doctor and clinic where you are planning prosthetics or implantation. It is also advisable to know what the already installed prosthetic structures are made of, and to communicate such nuances during subsequent prosthetics with another specialist.
The basic rule is to use the same metal for prosthetics. It is optimal to choose chemically inert metals - alloys of gold, platinum, palladium or titanium. And the metal prostheses or inclusions themselves must be manufactured using modern methods - casting or milling using CAD/CAM technology. Before prosthetics, it would be useful to take allergy tests to identify individual intolerance to metal - and if an allergy is detected, then it is better to choose ceramic, zirconium dioxide, acrylic non-monomer prostheses.
Don't know what type of prosthetics to choose?
We will help in the selection, advise where to read more information and compare types of prosthetics.
Consultation with an orthopedic doctor in Moscow clinics is free! Call now or request a call
Working hours: from 9:00 to 21:00 - seven days a week
Symptoms of the disease
The diagnosis of galvanosis a couple of months after the installation of prostheses begins to manifest itself with pronounced symptoms:
- persistent metallic taste;
- deterioration of taste sensitivity;
- increased sensations of acidity;
- burning tongue;
- soreness of the cheek mucosa;
- impaired salivation;
- migraine;
- irritability;
- insomnia;
- general weakness.
Symptoms appear in the morning and are often accompanied by disease of the oral mucosa:
- hyperkeratosis—keratinization of the mucous membrane;
- erosions and ulcers - thinning of the soft tissue structures of the oral cavity.
Classification and stages of development of galvanosis
Modern classification distinguishes 2 forms:
- Atypical. With this form, the conductivity of the salivary fluid, the current strength, and the number of active potential particles increase 4 times more than normal. The atypical form is a difficult pathology to diagnose. With prolonged neglect and failure to carry out timely treatment, growth of tumor-like formations, inflammation of the mucous membrane, and ulceration are possible.
- Typical. The potential increases over 4 times, accompanied by local and general symptoms. Often this form of pathology occurs in patients with destroyed and defective structures. Consequences: allergies to metals, malignancy of tumors and inflammation in soft tissues - gingivitis and papillitis. Possible damage to bone tissue.
Complications
The development of galvanosis is always accompanied by a decrease in the body’s general defense reactions. Electric currents formed during the interaction of prosthetic structures with saliva have a detrimental effect on the natural microbiological composition of the microflora of the oral cavity. All this together creates a favorable environment for inflammation to occur.
Often occur:
- gingivitis - inflammation of the gum tissue, leading to swelling and bleeding;
- papillitis - inflammation of the interdental papillae;
- stomatitis - developed under the influence of “heavy” metals, damage to the mucous membrane;
- herpes;
- frequent colds;
- activation of chronic processes.
If you do not pay attention to the primary symptoms of the pathology, and do not carry out prevention and treatment, the disease will be complicated by allergies and the formation of tumor-like formations.
Diagnostics
To identify galvanosis in dental centers, diagnosis is carried out using a detailed visual examination of the oral cavity using additional instruments; laboratory tests may be necessary.
The doctor identifies symptoms of failure to adapt to prostheses, disturbances in the structure of the device and its melting technology. The most important diagnostic method through which galvanosis is detected is measurement and evaluation examination.
- Measuring the potential difference between the structures and the salivary fluid with a voltmeter or ammeter. This is the main indicator of the pathological process.
- Measuring the pH of salivary fluid with an ionometer. We determine the saturation of saliva with hydrogen ions.
- Assessment of the composition of salivary fluid by spectral analysis.
In the presence of galvanosis, the potential difference in the mouth is up to 150 mV, and there is also a pH shift to an acidic environment.
It is important to be able to distinguish galvanosis from similar pathological processes:
- Glossalgia. Pain and swelling of the tongue and increasing viscousness of the salivary fluid, which is not typical for galvanosis.
- Lingual neuritis and trigeminal neuralgia. With these pathologies, touching the tongue causes sharp painful sensations.
- Stomatitis caused by toxic and allergic exposure. The digital data of the general blood test change - the ESR increases, and the blood cells increase slightly. This does not happen with galvanosis.
Electrochemical (corrosive) processes in the oral cavity
Saliva is a complex electrolyte , the composition of which depends on the general state of health, the condition of the oral cavity, and the presence of dentures.
The oral cavity is in a state of continuous aeration with each inhalation (excess oxygen) and carbonate saturation with each exhalation (excess carbon dioxide). It is an electrochemical system in which the role of the electrolyte is played by saliva (liquid phase), saturated with oxygen and carbon dioxide, and the role of electrodes is played by teeth and dentures (solid phase). At the boundary of the solid and liquid phases, a potential difference arises, or the potential φ of the electrode system, the value of which depends on the electrical conductivity of the solid phase (denture) and the concentration of potential-determining particles in the liquid phase (saliva). Metals have the greatest electrical conductivity, so the potential (φ) increases sharply if there are different metallic inclusions in the oral cavity (stainless steel, solder, amalgam, cobalt-chromium alloy and other combinations). In this case, short-circuited galvanic systems with different values of electrode potentials (φ) are formed in the oral cavity. Potential-determining particles are ions and molecules of substances that make up saliva, as well as gases (oxygen, carbon dioxide) that saturate saliva. However, since the concentration of potential-determining quantities in saliva is relatively small, changing it has practically little effect on the value of φ. To estimate φ, you can use the standard values specified in reference books.
Any electrode reaction can be represented as an equation:
An electrode reaction is a redox reaction that occurs at the boundary of a solid phase (which has electrical conductivity) and an electrolyte solution. Due to this reaction, a potential difference arises at the boundary of the solid phase (metal dentures, teeth) and electrolyte (potential of the electrode system φox/red).
The types of electrode systems are:
1. Emergence of one’s own potential:
2. The appearance of redox potential (redox potential):
In this case, electrons are transferred through a solid phase that is electrically conductive. A positive φ value indicates that Fe3+ is a strong oxidizing agent.
3. Emergence of gas system potential:
Examples of redox reactions at the boundary of the solid phase (metal prostheses) and liquid phase (saliva):
A positive value of φ° shows that chlorine and copper (Cu2+) are strong oxidizing agents.
A negative φ° value indicates that chromium and calcium are strong reducing agents.
1. The occurrence of a corrosive galvanic element in the presence of a gold prosthesis (at pH 5.5):
General electrochemical reaction:
2. The occurrence of a corrosive galvanic element in the presence of stainless steel prostheses:
3. Comparative anodic processes in an acidic and neutral environment of a metal prosthesis made of stainless steel.
The anodic portion of the prosthesis is dissolving.
Build-ups of poorly soluble Fe(OH)2 and Fe(OH)3 form on the anodic areas of the prosthesis.
In a neutral environment (pH 7.0), the electrochemical reaction is accompanied by an excess of hydrogen ions, i.e., increased acidity. This phenomenon is also confirmed clinically: patients with dentures made of stainless steel or cobalt-chromium alloy experience a feeling of acidity and burning in the oral cavity. It may subside somewhat or intensify during meals (plant foods create an acidic environment, protein foods create an alkaline environment). Apparently, such patients should be recommended protein foods to neutralize excess hydrogen ions.
In an acidic environment, a pronounced process of dissolution of the metal prosthesis—the anodic areas—occurs.
Thus, anodic corrosion reactions of dentures are characterized by a change in electrode potentials due to the transition of metal ions from the solid phase (metal dentures) to the liquid phase (saliva). These provisions were confirmed by us in an experiment and in an orthopedic dentistry clinic.
Stainless steel, proposed in the 1930s for dental prosthetics, was tested for corrosion resistance. D.N. Tsitrin and V.N. Dyatlova (1934) determined the degree of corrosion, taking into account the loss of mass and a change in the appearance of the tested part. The mass loss was extremely insignificant and was determined by the gravimetric method. The appearance of the samples did not change. Based on these data, the authors concluded that stainless steel is a satisfactory alloy for dental prosthetics.
However, it is known from electrochemistry that corrosion is also determined by the quality and quantity of components released into the environment from the tested (samples) metal alloys. We have developed and applied a method of chemical spectral analysis to determine corrosion processes in an artificial environment [Gozhaya L.D., 1969]. When creating an artificial environment, we were guided by literature data on the chemical composition of human saliva.
The artificial environment was placed in a device (Fig. 1), which was a quartz communicating vessel covered with a thermal insulation layer to maintain a constant temperature. Heating (37° C) of the liquid in the vessel was carried out by electric current supplied under the heat-insulating layer through a laboratory autotransformer TIP-1. In one elbow of the vessel there was a stirrer for mixing and connecting to an electric motor, in the other there was a stand for test samples, and at the base of the vessel there was a tap for sampling. The vessel was closed with fluoroplastic stoppers. The total amount of artificial medium was 1000 g. For analysis, 8 cm3 of the test liquid was taken, divided into two samples (4 cm3) and the average value was determined. Sampling was carried out every 6 days. 15 samples were taken, 30 analyzes were carried out, 150 spectrograms were studied. Spectral analysis revealed corrosive changes in the test environment. The corrosion study was carried out at a pH of 5.5 (the maximum pH shift possible in the oral cavity) and at a temperature of 37°C. For corrosion testing, three stainless steel bridges with solder were taken (weight before the experiment 6.92 g, after the experiment 6.86 g), the loss was 0.06 g (0.87%). Test time 3 months (2100 hours). During this time, the qualitative and quantitative characteristics of the corrosion process were determined.
Upon examination (before the experiment), the bridges in the soldering areas had darkening due to the formation of oxide products, the outer surface was polished and shiny. Before the start of the study, the bridges were carefully polished. The inner surface of the crowns is matte. After the experiment, in the places where the crowns and the intermediate part were soldered, the surface was green-blue; an oxide film in the form of “growths” was formed, after removing which crater-shaped depressions (“ulcers”) were discovered.
Mechanical fracture in these places led to crown fracture. The surface of the polished part of the bridges became dull, and a dark yellow oxide film appeared inside the crowns. These phenomena can be explained by the corrosion process.
According to D. G. Tufanov (1969) and M. Andreas (1960), in aggressive environments, stainless steel with solder is subject to contact corrosion. In this case, the ratio of the areas of the contacted metals is of great importance.
The combination of a large cathode surface (stainless steel) with a small anode surface (solder) causes significant dissolution of the metals - the solder.
In Fig. Figure 2 shows the dependence of the difference in blackening (S) of microimpurities of iron, copper, nickel, chromium on the test time (T) of stainless steel with solder in an artificial environment.
From Fig. 2 shows that the content of analyzed impurities in the artificial environment increases with increasing test time. This dependence is especially pronounced for manganese, iron, copper, nickel, and less so for chromium.
This is due to the different chemical reactivity of these metals, determined by different electrochemical potentials:
Mn(-1.19 V) Ni(-0.23 V)
Cr (-0.91 V) Cu (+ 0.34 V)
Fe (-0.44 V) Ag(+0.8 V)
Chromium is easily passivated, that is, it is covered with a very dense phase of Cr2O3, and becomes a low-active metal.
φо = + 0.4 V
Copper in the presence of sulfur (S-2) and other sulfides (Na2S; K2S) forms CuS and becomes an active metal.
The nature of the curves shows that corrosion has temporary stages of activation and deceleration.
Thus, according to spectrographic studies, stainless steel with solder in an artificial environment close to the conditions of the oral cavity is subject to corrosion.
4 samples of silver-palladium alloy (special alloy) weighing 1.5028; 1.5692; 1.5519 and 1.3822 g (total mass 6.001 g). The mass of the tested special alloy samples did not change after the experiment. Test time to clarify the passivation process is 5 months. Sampling for the study was carried out every 6 days during the 1st month, then every 2 weeks. 12 samples were taken, 24 spectral analyzes were carried out.
In Fig. Figure 3 shows that during the 1st month the silver content in the test medium increases significantly (from 9*10-6 to 2*10-5%). Then the release of silver slows down somewhat. This phenomenon can be explained by the initial effect of a weakly acidic environment on the transition of silver from the special alloy into the environment, followed by passivation (formation of an oxide film on the surface of the test samples).
Corrosion increases with increasing test time. The main component, silver, corrodes.
Analysis taking into account potentials [Lurye Yu. Yu., 1983] shows that the potentials of silver decrease in the presence of substances containing chlorine, bromine ions, ammonia molecules, etc. In this case, the emf of the silver-palladium alloy should increase, and the corrosion process should be activated . However, in slightly acidic and neutral environments, the potential of silver increases sharply in the presence of oxygen (can reach 2 V). In this case (breathing mainly through the mouth), the EMF of the corrosive galvanic cell decreases and a temporary attenuation of the corrosion process or a redistribution of the cathode and anodic sections is observed, i.e., palladium temporarily becomes the anodic section and hard or soft growths with great forces can form on its surface adhesion to the palladium surface.
Anode (Ag) Cathode (Pd)
φ = +0.8 V φ= +0.99 V
Contact corrosion occurs, as a result of which the silver corrodes (since φAg<φPd).
Chemical spectral analysis is a reliable test for assessing the corrosion resistance of metal alloys in biological environments. In model experiments using chemical-spectral analysis, it was shown that the galvanic couple gold - cobalt chromium corrodes and leads to the accumulation of chromium ions (2 * 10-5%), nickel (5 * 10-7%) in artificial saliva (pH 5.5). , iron (3 * 10-5%) - Iron is included in the cobalt-chromium alloy in the amount of 0.5%, chromium - 25-28%, nickel - about 4%. In the model of saliva pH 7.0 and 8.0, chromium and nickel ions are not detected, and iron ions are present in a concentration of 1-10-5% [Sedov S. E., 1983].
Clinical studies of biological media (saliva, blood, urine, mucous membranes, etc.) for microelements in individuals with metal structures in the oral cavity confirm model experiments and reveal the processes of corrosion of the prosthesis in the oral cavity.
The products of electrochemical reactions are metal ions (microelements) entering saliva from corroding alloys. The dynamics of changes in microelements in the composition of saliva is directly dependent on the degree of electrochemical processes in the oral cavity. We found high concentrations of microelements in the saliva of persons with allergic and toxic stomatitis caused by a stainless steel prosthesis (400 people), compared to the norm (50 people). The most pronounced fluctuations were found in the content of iron, nickel, copper, silver, chromium, and titanium.
Changes in the quantitative content of microelements in saliva are closely related to the processes of corrosion of stainless steel bridges. We established this when examining a removed soldered stainless steel prosthesis after working it in the oral cavity. For this purpose, 30 people aged from 35 to 58 years were examined. The first group consisted of 8 patients whose bridge-like prostheses were removed due to separation of the intermediate parts at the soldering points (10 prostheses). The second group included 22 patients with oral paresthesia (16) and allergies to metal dentures (6). In these patients, the prostheses were removed after establishing a causal relationship between the morbidity and the metal structures. More than 35 prostheses were removed and examined. The diagnosis of the disease was made based on data from clinical and laboratory studies (spectral analysis of saliva, blood tests, allergy tests).
The removed stainless steel bridges were examined on a scanning electron microscope using a microprobe analyzer. In each prosthesis, three zones were studied: solder, solder-to-crown contact, and solder to intermediate (cast) part. A total of 90 areas of bridges were examined, and 30 spectral analyzes of saliva were performed.
When examining the solder in patients of the first group, a network of corrosion cracks and intercrystalline corrosion were found (Fig. 4), which indicates the simultaneous action of mechanical stress and a corrosive environment. As is known, intergranular corrosion occurs under the combined action of an aggressive environment and internal stresses [Kabanov B.N., 1966]. In the oral cavity, dentures are largely susceptible to electrochemical corrosion, intensified due to the interaction of dissimilar metals (steel - solder). In patients in this group, a large length of bridge-like prostheses was noted, built without taking into account the periodontal condition of the supporting teeth and antagonist teeth. In this case, stresses in the metal enhance the corrosion process. Increased corrosion is also facilitated by the presence of gaps due to poor-quality welds, causing crevice corrosion [Zhuk N. T., 1976]. Violation of the soldering temperature in the range of 450-850 ° C leads to intergranular corrosion. In this case, carbides are released along the grain boundaries [Gerner M. M. et al., 1984].
In the area of contact between the solder and the crown, corrosion cracking and mechanical destruction are observed (Fig. 5). In the cast part of the bridge (third zone), the corrosion process is very weakly expressed. When examining patients of the second group, the bridge-like prostheses in the places of soldering (solder) had thick, large-area, porous oxide films. It is known that only very thin passivating layers resist corrosion [B. N. Kabanov, 1966]. After their removal, the bridges were destroyed mechanically at the crown-solder interface.
When examining the fracture zone using a scanning electron microscope, several large pores were discovered. Surface pores communicating with the oral environment and pores inside the seam (solder) contained corrosion products and were the source of crevice corrosion (Fig. 6). The structure of the solder is porous, its surface coincides with the components of the silver-copper eutectic. However, the nature of the distribution of copper and manganese inclusions in the solder is not always uniform: there are large and small accumulations of manganese, copper, and areas where inclusions are absent (Fig. 7). Silver inclusions are distributed evenly in the solder (Fig. 8). The presence in the structure of individual microregions rich in copper is characterized by reduced corrosion resistance. It is also known that inclusions determine the severity of electrochemical reactions in the oral cavity, since their electrode potentials are different.
Thus, stainless steel dentures are subject to corrosion in the oral cavity. Crevice corrosion is observed in the surface pores of the solder. Its products (microimpurities of iron, copper, manganese, silver, etc.) enter the oral cavity, which is confirmed by spectral analysis of saliva. This process is constant over time. Corrosion occurs throughout the entire depth of the solder. In the area adjacent to the crowns, the solder corrodes to a lesser extent. In the cast steel structure, corrosion is weakly expressed. The occurrence of intergranular corrosion is facilitated by mechanical stresses in the metal, significant length of the prostheses, violations of the configuration of the soldered seam and the formation of gaps.
Bridges with intercrystalline corrosion in the oral cavity can collapse along the seam (soldering).
Treatment
Therapeutic measures to eliminate galvanosis should be a combination of the work of related specialists and include important stages:
- metal structures should be removed from the oral cavity as a priority;
- elimination of local inflammatory and allergic reactions;
- strengthening the body's immune defense.
Before starting treatment, the doctor completely examines all components of the prosthesis and identifies problem areas. The discomfort-producing structure is removed from the oral cavity. Also, an important stage in the treatment of galvanosis is the treatment of inflammatory processes of the mucous membrane. Local and general immune-strengthening therapy drugs are prescribed.
After treatment, the symptoms of the underlying disease disappear within a week, all indicators return to normal.
Galvanic syndrome in the mouth - why it appears and how to deal with it
Article navigation
- What is galvanic syndrome
- Causes
- Classification and symptoms
- Diagnostics
- Treatment
- Complications without treatment
- Prevention
- Cost of treatment
- User Questions
Question for a specialist
Dental prosthetics is sometimes complicated by some rather unpleasant conditions - and a person may not even suspect that the problem is in the prosthesis. One of these processes is galvanism in the oral cavity, which is also called “galvanosis” or “galvanic syndrome”. Next, we will tell you why it occurs, what symptoms it is accompanied by, how it is treated - and whether it is worth doing anything about it at all.
Preventive actions
With correctly performed diagnostic and therapeutic measures, patients have everything they need to fully restore chewing function after replacing prosthetic structures with hypoallergenic ones.
Preventing pathology is much easier than full-scale therapeutic methods. Therefore, prevention is an important stage in caring for one’s health for every patient. In order not to miss the disease at the very beginning, when everything can be corrected as gently as possible, it is important for a patient with orthopedic structures to visit an orthopedic dentist 2 times a year to perform a preventive examination, as well as perform a professional examination. hygiene and sanitation of the oral cavity.
Specialists from West Dental clinic branches will always help identify the problem in a timely manner and offer comprehensive, appropriate treatment.