Endoscopic interventions are minimally invasive, highly informative and effective medical services aimed at diagnosis and treatment (endoscopic manipulation, including endoscopic surgery) of various diseases. Today it is impossible to imagine modern oncology, gastroenterology, therapy, urology, pediatrics, traumatology without modern endoscopy. In the article we will pay attention to compliance with sanitary and epidemiological requirements for endoscopy rooms.
General sanitary and epidemiological requirements for organizations engaged in medical activities are contained in SanPiN 2.1.3.2630-10[1]. This document, in particular, provides requirements for placement, design, equipment, maintenance, anti-epidemic regime, preventive and anti-epidemic measures, working conditions for personnel, catering for patients and personnel of organizations carrying out medical activities. Special sanitary and epidemiological requirements for endoscopic interventions are given in SanPiN 3.1.3263-15[2]. Let us remind you what requirements are established by this document.
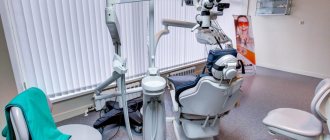
Structure, composition, technical features of premises.
In accordance with clause 5.1 of SanPiN 3.1.3263-15, the endoscopy department (office) must have the following premises:
1) doctor's office;
2) separate endoscopic manipulation zones (depending on the types of interventions performed) for:
- bronchoscopy; studies of the upper gastrointestinal tract;
- studies of the lower gastrointestinal tract;
3) washing and disinfection room;
4) auxiliary premises.
To conduct studies of the lower parts of the digestive tract, a sanitary facility must be provided (clause 5.3 of SanPiN 3.1.3263-15).
It is important to note that the manipulation room for bronchoscopy belongs to cleanliness class B; this room is equipped with a supply and exhaust ventilation system with a predominance of air flow. The supplied air must be cleaned and disinfected with an efficiency of at least 95%. The room for processing endoscopes should be equipped with general supply and exhaust ventilation and local exhaust ventilation with removal of solution vapors at the level of the washing baths.
The room in which endoscopic interventions are performed must be equipped with a sink for washing the hands of medical workers, with hot and cold water supply, including a backup hot water supply.
Note:
The location of technological equipment in the room for processing endoscopes must ensure the flow of all stages of processing endoscopes in accordance with the requirements of sanitary rules. In newly designed medical organizations, planning solutions should be provided that eliminate the cross-flow of clean and dirty endoscopes. For existing offices, the endoscope processing room is functionally divided into a conditionally dirty area, intended for final cleaning, and a conditionally clean area, where high-level disinfection, drying and storage of endoscopes is carried out. A sink is installed in the endoscope processing room for medical personnel to wash their hands. It is not permitted to be used for other purposes.
Area of responsibility during interventions.
In accordance with clause 3.2 of SanPiN 3.1.3263-15, in each structural unit of a medical organization performing endoscopic intervention, persons responsible for organizing and carrying out anti-epidemic measures, including the quality of processing of endoscopic equipment, must be identified.
The head (doctor) of the structural unit (office) performing endoscopic interventions must develop work instructions for processing endoscopes available in the equipment of the structural unit (office), which is approved by the head of the medical organization. The specified instructions must be drawn up on the basis of the provisions of these sanitary rules, taking into account the type, brand (model) of endoscopes, operational documentation for them and equipment intended for their processing and storage, instructions for the use of chemical cleaning, disinfection and sterilization agents used (clause 3.3 of SanPiN 3.1.3263-15).
Doctors directly involved in performing endoscopic interventions and processing endoscopic equipment must undergo advanced training at least once every five years on the basis of organizations licensed to conduct educational activities under programs of additional professional education, including issues of ensuring the epidemiological safety of endoscopic interventions (clause 3.4 SanPiN 3.1.3263-15).
Each endoscope equipped at a structural unit in which endoscopic interventions are performed is assigned an identification number, which includes information about its type, model and serial number. The identification number of the endoscope used during a medical intervention must be indicated in the endoscopic intervention protocol, in the special notes column of the journal for recording studies performed in the endoscopy department, or in the journal for recording surgical interventions (clause 3.6 of SanPiN 3.1.3263-15).
Gosopzhnadzor requirements
The requirements of this organization will depend on whether you are equipping an x-ray room or not. Usually, in small dental offices, such equipment is not available. This structure imposes requirements both on the premises and organization of fire safety measures (fire safety), and on documentation (the availability of orders, safety instructions, magazines, signs and reminders).
You can prepare most of these documents yourself or order a ready-made package from an organization that provides similar services.
Regulations
- No. 123-FZ of the Russian Federation (Technical regulations, including Art. 82).
- SNiP 31-01-2003/SNiP 31-02 (for blocked buildings, except mobile ones).
- RD 78.145-93 (installation of fire and security alarms).
- NPB 110-03.
- PPB 01-03.
- SNiP 21-01-97 (updating SP112.13330.2011).
Requirements for the room and its decoration
According to fire safety requirements, the finishing of premises is carried out with materials that are not amenable to combustion:
- water-based paints;
- tile.
If your office is located on the 2nd floor of a residential building, the flight of stairs must be at least 1.2 meters wide. It is advisable that the door of your room opens outwards. It is prohibited to obstruct the exit with any objects.
Documentation requirements
To organize any form of ownership, it is mandatory to have:
- TB instructions.
- An order to appoint a person responsible for safety and security, to inspect the premises at the end of the working day and before starting up the installations.
- Logbook for safety briefings.
- Personnel knowledge testing log.
- Logbook for registration of inspections by regulatory authorities.
- Logbook of primary fire extinguishing equipment and fire extinguisher maintenance.
- Plates indicating fire hazards for electrical equipment.
- Plates with the name of the person responsible for compliance with the fire safety regime and the fire service call number.
- Colored evacuation plan in A3 format.
Wiring Requirements
The wiring and grounding loop are done by a licensed organization. Testing of the grounding system is also carried out by a specialized organization or by an employee who has the right to carry out special work of this kind. Such tests are mandatory (according to PP No. 291 dated 04/16/12). Periodic grounding checks are also mandatory.
When calculating the number of sockets, please note that air disinfecting lamps (bactericidal) and, if possible, recirculating units must be installed in the office.
Requirements for fire fighting equipment
The dental office must have primary fire extinguishing equipment. First of all, fire extinguishers, at least two. Their number depends on the area of the room. Fire extinguishers must be entered in a logbook, checked, must have a tag with the date of verification and instructions for their use. They should be located in an easily accessible place.
The dental office must have a fire alarm system. Usually non-addressable systems are used, the requirements for them are minimal and they successfully serve small areas. Such a system must be installed and maintained by a licensed organization.
For small dental clinics (3-4 offices) it is enough to use the “Signal-10″+SOUE” model system; for larger clinics it is better to use PPK-2 with type 3 sounders with the system connected via TRV-1x2x0.5 (wires), SVV-2x0.5/SVV-6x0.5 (cables).
Personnel requirements
Personnel must be knowledgeable about safety regulations, know the rules for disconnecting/connecting equipment, and not use faulty installations or broken sockets.
All personnel must:
- undergo training on industrial safety (introductory, primary, regular) with a record of this in the journal and a knowledge test;
- be able to use fire extinguishing means and know where they are located;
- Know your actions in case of fire, be able to help clients evacuate.
Before opening an organization, check the relevance of the requirements with your local regulatory authorities.
Requirements for health protection of medical personnel.
Medical workers of structural units of a medical organization performing endoscopic interventions must undergo preliminary and periodic medical examinations (clause 12.1 of SanPiN 3.1.3263-15). Also, medical personnel of structural units of a medical organization performing endoscopic interventions must be vaccinated against infectious diseases in accordance with the national calendar of preventive vaccinations (clause 12.2 of SanPiN 3.1.3263-15).
Before being allowed to work related to performing endoscopic interventions or processing endoscopic equipment, each medical worker is required to undergo special primary training on the rules for processing endoscopes and workplace health instruction.
All medical personnel of structural units performing endoscopic interventions must be provided with medical clothing (gowns, surgical suits, caps, masks) in accordance with the equipment sheet (at least three sets per worker) and personal protective equipment in sufficient quantities.
The head of the medical organization is responsible for providing medical workers with medical clothing and personal protective equipment (clause 12.4 of SanPiN 3.1.3263-15).
A change of medical clothing (gown or pajamas, cap) for the staff of the department (office) inside the luminal endoscopy should be carried out as it gets dirty, but at least twice a week, for the personnel of the surgical (endoscopic) departments (offices) performing surgical endoscopic interventions - as pollution, but at least once a day (clause 12.5 of SanPiN 3.1.3263-15).
Before performing each non-sterile endoscopic intervention, the personnel involved in it perform hand hygiene in accordance with the requirements of SanPiN 2.1.3.2630-10[3] and put on personal protective equipment (disposable mask, goggles, disposable medical gloves, waterproof gown or disposable apron ).
Before carrying out each sterile endoscopic intervention, the personnel involved in it clean their hands according to the method of treating surgeons’ hands in accordance with the requirements of SanPiN 2.1.3.2630-10, put on a cap, mask, sterile gown and gloves.
To reduce the risk of infection of personnel and ensure the reliability of processing flexible endoscopes for non-sterile intervention, a mechanized method using MDM is used. With a large turnover of endoscopes (simultaneous processing of three or more endoscopes of the same type), a mechanized method of processing endoscopes is mandatory.
To prevent injuries from instruments to endoscopes with piercing surfaces, it is necessary to minimize personnel contact with untreated instruments using containers with perforated inserts, MDMs and ultrasonic cleaners. It is prohibited to use injection needles to collect pathological material from biopsy forceps.
Cases of injury to medical personnel at all stages of preparation for sterilization of instruments for endoscopes with piercing-cutting surfaces must be recorded in the logbook of injuries and emergency situations.
Why does the current Order become invalid?
Until the end of 2022, Order of the Ministry of Health and Social Development of the Russian Federation dated December 7, 2011 No. 1496n “On approval of the Procedure for providing medical care to the adult population for dental diseases” is applied. From January 1, 2022, this order will no longer be in force for a number of reasons.
The first reason is the publication of Decree of the Government of the Russian Federation dated June 17, 2020 No. 868. This resolution, in turn, was issued within the framework of the so-called “regulatory guillotine”.
The “regulatory guillotine” is a mechanism aimed at creating a modern and adequate regulatory system based on identifying and regulating the most significant public risks.
The mechanism is aimed at eliminating the excessive requirements of public authorities imposed on organizations of various forms of ownership, and introducing new updated requirements based on a risk-oriented approach to the activities of organizations.
In other words, the “regulatory guillotine” aims to eliminate redundant requirements and make it easier for organizations to comply with legal requirements.
The second reason is the activity of specialized associations that insisted on updating the requirements and, in particular, the standards for equipping dental clinics. Thus, the equipment standards prescribed by the procedure for providing medical care have become significantly outdated after almost 10 years of use. It was necessary to update the lists of necessary equipment and bring them into compliance with other regulations.
Accordingly, it can be assumed that the new order of the Ministry of Health, approving the Procedure for providing medical care to the adult population for dental diseases, should contain more harmonious requirements for dental clinics and their employees. In fact, this Procedure in some places raises questions from the professional community. Let's consider its provisions.
Sanitary measures in the endoscopy room.
Special requirements are imposed on sanitary measures carried out in the endoscopy room. Thus, in accordance with clause 5.16 of SanPiN 3.1.3263-15, cleaning and preventive disinfection in manipulation rooms for non-sterile endoscopic interventions, in the washing and disinfection room should be carried out as they become dirty, but at least once per shift or twice a day. After each patient, the surface of the couch must be disinfected. General cleaning should be done once a week.
All types of cabinets for storing processed endoscopes must be cleaned and disinfected with a chemical solution in a bactericidal mode at least once a week, unless otherwise provided in the operating instructions (clause 5.15 of SanPiN 3.1.3263-15).
Preliminary cleaning of used endoscopes and instruments for them is carried out in the same room where the intervention was carried out (clause 5.6 of SanPiN 3.1.3263-15). Final cleaning (final cleaning combined with disinfection) and high-level disinfection of endoscopes intended for non-sterile endoscopic interventions are carried out in a specially equipped washing and disinfection room or room for processing endoscopes (clause 5.7 of SanPiN 3.1.3263-15).
When processing endoscopes and other medical products as part of endoscopic and endosurgical complexes, as well as instruments for endoscopes, medical equipment products, sterilizers, washing machines, ultrasonic cleaners, detergents and disinfectants approved for use for these purposes in the Russian Federation must be used.
It is extremely important that when choosing cleaning, disinfection, as well as sterilization products and methods, the recommendations of manufacturers of endoscopes and instruments for them regarding the impact of a particular product on the materials of these medical devices should be taken into account. It is not allowed to use disinfectants for cleaning or cleaning combined with disinfection, which in the recommended modes have a fixing effect on organic contaminants, including those containing alcohols and aldehydes (clause 7.3 of SanPiN 3.1.3263-15).
New Procedure for providing medical care to adults for dental diseases
Let's look point by point at the Order of the Ministry of Health of Russia dated July 31, 2020 No. 786n “On approval of the Procedure for providing medical care to the adult population for dental diseases” (hereinafter referred to as the New Order) in comparison with the current Order of the Ministry of Health and Social Development of the Russian Federation dated December 7, 2011 No. 1496n “On approval of the Procedure for providing medical care to the adult population for dental diseases” (hereinafter referred to as the Old Order).
So, according to the Old Order, medical care is provided to the adult population for dental diseases of the teeth, periodontium, oral mucosa, tongue, salivary glands, jaws, face and head, including:
- carious, non-carious and other dental lesions;
- acute, chronic and specific inflammatory diseases, acute and chronic trauma, acquired defects and deformations, oncological diseases of the periodontium, oral mucosa, tongue, salivary glands, jaws, face and head;
- anomalies and defects in the development of teeth, jaws, face and head, their prerequisites and consequences.
The new order contains a similar provision, but the mention of “face and head” has disappeared from it. In principle, the parts devoted to maxillofacial surgery are excluded from the New Order, since for this area of medical activity a separate order of the Ministry of Health of Russia dated June 14, 2019 No. 422n “On approval of the Procedure for the provision of medical care in the profile of “maxillofacial surgery” is provided. Thus, from 2022, the existing duplication of regulations governing the activities of maxillofacial surgeons will be eliminated. When providing medical services in the field of maxillofacial surgery, it is the Procedure approved by Order No. 422n that will be mandatory to comply with.
The New Order also reduced the number of types of medical care:
| Old order | New order |
| Medical care for the adult population for dental diseases is provided in the form of: | |
|
|
That is, the New Order no longer provides for this type of medical care for the adult population in the “Dentistry” profile, such as emergency medical care.
Instead, the New Order contains instructions on the forms of medical care for dental diseases:
- emergency form (provided for sudden acute diseases, conditions, exacerbation of chronic diseases that pose a threat to the patient’s life);
- emergency form (provided for sudden acute diseases, conditions, exacerbation of chronic diseases, without obvious signs of a threat to the patient’s life);
- planned form (provided during preventive measures, for diseases and conditions that are not accompanied by a threat to the patient’s life, do not require emergency and urgent medical care, and delaying the provision of which for a certain time will not entail a deterioration in the patient’s condition, a threat to his life and health).
As a positive innovation, we can note the inclusion in the text of the Procedure of brief definitions of emergency and emergency forms of medical care, since in practice the distinction between these forms raises a number of questions. The New Order also contains generalized medical indications for the provision of medical care in the form of emergency and emergency medical care, which are inflammatory diseases of the oral cavity, including the oral mucosa, tongue, salivary glands of various etiologies and localizations.
Let us consider the conditions for providing medical care to the adult population for dental diseases.
In accordance with the New Order, medical care for dental diseases can be provided on an outpatient basis and in a day hospital:
| Conditions for providing medical care | Criteria |
| outpatient | in conditions that do not provide round-the-clock medical supervision and treatment |
| in a day hospital setting | in conditions that provide medical supervision and treatment during the day, but do not require round-the-clock medical supervision and treatment |
Thus, the possibility of treating dental patients in a day hospital will allow doctors to monitor patients when necessary, for example, if they are elderly people with concomitant chronic diseases.
Let's sum up the intermediate results. In accordance with the New Order, medical care for the adult population for dental diseases is provided within the following criteria:
| Type of medical care | Conditions | Forms |
| Primary health care | Outpatient | Emergency |
| Specialized | Day hospital | Urgent |
| Planned |
Requirements for the endoscope processing cycle.
In accordance with clause 4.1 of SanPiN 3.1.3263-15, endoscopes for non-sterile endoscopic interventions and their accessories (valves, plugs, caps) immediately after use are subject to the following:
- pre-cleaning;
- final cleaning (final cleaning combined with disinfection);
- high level disinfection;
- storage under conditions that exclude secondary contamination.
Endoscopic equipment, including endoscopes, for sterile endoscopic interventions, all types of instruments for sterile and non-sterile interventions immediately after use are subject to the following (clause 4.2 of SanPiN 3.1.3263-15):
- pre-cleaning;
- pre-sterilization cleaning combined with disinfection;
- sterilization;
- storage under conditions that exclude secondary contamination.
Immediately after each use of an endoscope intended for non-sterile interventions, all stages of its reprocessing must be completed in full. All channels of the endoscope are processed, regardless of whether they were involved in endoscopic intervention or not.
The process of sterilization of endoscopes and instruments for them can be transferred to the next work shift, provided that they are effectively disinfected and pre-sterilized cleaned immediately after use.
Note:
In accordance with clause 3.7 of SanPiN 3.1.3263-15, each endoscope processing cycle must be recorded in logs.
In addition, it should be noted that sterilizers are subject to bacteriological control after their installation (repair), as well as during operation at least twice a year as part of production control (clause 2.36 of SanPiN 2.1.3.2630-10). Maintenance, warranty and current repairs of sterilizers are carried out by service specialists (clause 2.37 of San Pin 2.1.3.2630-10).
Rospotrebnadzor requirements
Keep in mind when opening an office that, according to the requirements of the SES, the dentist has the right to work only with the participation of nursing staff, who prepare and disinfect workplaces and equipment.
Regulatory documents
The main document to be followed when opening a dental office is SanPiN 2.1.3.2630-10. It contains all the important points regarding the choice of the territorial location of the office, its premises, its decoration, installation of utility networks and personnel hygiene. You can get additional information from SanPiN 2956a-83.
The second document, according to the list, and not in importance, that must be taken into account and “on which” the SES commission will strictly ask, is the POZPP.
There are many GOSTs and SanPiNs that water and sewerage, lighting and microclimate in the work area must comply with. But SanPiN 2.1.3.2630-10 takes into account most of their requirements. Therefore, it is better to start getting acquainted with the regulatory documentation with it.
Before looking for a base for your office, check to see if your region has rules developed specifically for small dental businesses.
Requirements for office location
The SES will have quite strict requirements for the placement of a clinic with a hospital, an X-ray room and its own sterilization room.
But if you open a small office, you can place it in almost any territory:
- on the first and second floors of buildings designed for housing;
- in a separate block or permanent structure;
- in a built-in room or attached outbuilding.
If you purchase a residential apartment for your office, you will need to convert it to non-residential. This is a rather lengthy procedure and you may need coordination not only with the fire department and SES, but also with:
- Housing association.
- Protection of architectural monuments.
- An environmental organization that may require you to landscape the surrounding area.
- And even the traffic police, they may insist on organizing parking spaces for clients near your office.
You can even rent an office with equipment from a municipal medical institution. This will greatly simplify your cooperation with regulatory authorities.
If you are located in a residential building, you must arrange a separate exit for your office.
Premises requirements
When choosing a room, you will, of course, have some restrictions. For example, you need to select rooms:
- with ceilings from 2.6 m;
- with existing utility networks, including drinking water supply (this means that institutions provided only with technical water are not suitable for you as landlords);
- with an area of at least 14 m2 +10 m2 for each additional workplace or +7 m2 for each chair not equipped with a drill.
In addition, it is recommended to highlight:
- 10 m2 for a place to wait for visitors, a wardrobe and a place for a medical administrator.
- And also 3 square meters for the toilet room.
- Staff room with dressing room (6 m2).
- Pantry - 3 m2.
For the number of seats up to 3 pcs. It is allowed to make one bathroom for staff and clients. If there are more chairs, you will have to arrange a separate toilet room for clients. You can see in detail the requirements for the area of dental offices in Appendix No. 2 to the above SanPiN
.
The set of premises that a dental office must have depends on the type of services provided, what you plan to license and on the number of chairs to be installed.
If you plan to receive children, this is done in a separate block with a separate installation and toilet. It is prohibited to accommodate children and adults on a schedule using the same equipment.
If you are planning to provide surgical services, you need to prepare a separate room for this, dividing it into two zones: “purulent” and “clean”.
Finishing requirements
All surfaces of dental offices must be flat, smooth, easy to clean and covered with materials that do not deteriorate from frequent contact with disinfectants. The joints between floors and walls should be rounded, without gaps where dust and dirt can get clogged. The floor in the office itself can even be linoleum, but the edges of the linoleum are “run” under the baseboard, the joints of the panels are soldered.
The bathroom, walls around the sink and equipment, the operation of which can lead to moisture in the walls, are finished with tiles.
Moreover, the sink and cabinet walls:
- to a height of 1.6 meters from the floor;
- 0.2 meters beyond the appliance or sink.
Ceilings can be suspended, suspended, etc.; the main requirement for them is that they must be easy to clean and allow the possibility of disinfection.
The colors you choose to paint your cabinet should be neutral and light. This is justified by the fact that their coating should not interfere with the doctor’s perception of the color of teeth, enamel, gums or blood.
If you plan to work with mercury-based amalgam, you can cover the walls with plaster (brick) or grout (panels) with an admixture of 5% sulfur in the finishing material to bind mercury fumes. No decorations on the walls of such an office are allowed.
Requirements for the microclimatic conditions of the dental office
Working conditions for doctors are ensured by proper ventilation and heating. The heating system must be autonomous and maintain air cleanliness (including in terms of contamination of the air with pathogenic microorganisms) in accordance with the maximum permissible concentrations specified in SanPiN.
Temperature and humidity indicators must be within the limits:
- not lower than +18°C in winter, not higher than +25°C in summer;
- rel. ow. — from 40 to 60%;
- speed of movement of air masses - 0.2 m/s.
Maintaining comfortable conditions in dentistry less than 500 m2 can be done using:
- natural (window) ventilation (for this, appropriate transoms must be installed and easy access to them must be made);
- organizing the supply and exhaust system;
- use of split systems approved for medical institutions (in this case, it is necessary to clean the filters every six months).
The laying of utility networks in dental offices is carried out hidden. If there are any malfunctions in the ventilation system, they must be corrected immediately. The air must comply with SanPiN in terms of microbiological and chemical indicators. At the same time, the flow of air from “polluted” zones to “clean” ones is considered unacceptable. This must be taken into account at the ventilation planning stage.
Requirements for water supply and sewerage
The dental office must have hot and cold water supply. Water must be supplied by flow method. In the absence of a centralized water supply, it is allowed to obtain water from your own source if you have a permit from the SES.
Wastewater is treated in city-wide facilities, but if there are none, then complete biological treatment and disinfection is carried out in local facilities, which must be provided for (Resolution of the Chief Doctor of Sanitary Service No. 76).
equipment requirements
When selecting and installing equipment, you must be guided by the above standards and SanPiN 2.6.1.1192-03 (if you are planning to install X-ray equipment).
If the office is equipped in a room with one-way natural (sun) light, then all chairs are mounted along one wall (light-carrying). Opaque partitions of at least 1.5 meters between seats are required.
The dental office must have equipment for sterilizing instruments and sinks:
- or two-section;
- or separate.
One sink (compartment) is used for washing the hands of medical workers, the other is used for processing equipment.
Air disinfection equipment, including bactericidal lamps, must be installed in offices.
If you plan to work with gypsum material in your office, you must ensure that you have gypsum traps that precipitate this substance from the wastewater.
The level of noise and vibration indicators during the operation of dental office equipment must comply with the standards approved by the Resolution of the Chief Sanitary Doctor No. 58 of 05/18/10 (as amended by No. 76 of 06/10/16), specified in Appendix No. 9 to SanPin.
Please note that the box in which the chair's connections to the sewerage system, water supply, electrical network, vacuum line and compressed air are mounted is mounted at each dental chair. Such a box is installed no further than 50 cm from the workplace.
Lighting: basic requirements
All dental offices must have both natural and artificial lighting. It is better to choose a room with windows oriented to the north side, if this is not possible, light protection devices are installed on the windows:
- visors;
- easy to clean blinds;
- special films (installed between the glass units).
Lamps used to illuminate dental offices should not distort color rendition. In this case, it is necessary to place the lamps so that they do not fall into the dentist’s field of vision during his work (there are general lighting lamps). Local illumination of the doctor's workplace is mandatory for dental offices (for a surgeon - shadowless).
All illuminators must be easy to clean, the luminaires must have explosion-proof fittings and must not allow personnel to be blinded during operation.
Requirements for medical personnel
Medical work of a dental office must have:
- V/O and S/O of medical orientation;
- take qualification courses every 5 years and have certificates confirming the success of passing exams;
- a medical record, including the dates of medical examinations and courses to improve the level of sanitary knowledge.
All personnel (from doctors to nurses) must wash their hands thoroughly and comply with the following conditions:
- cut your nails short (extended nails or painted nails are not allowed);
- refuse to wear jewelry on your hands while working;
- surgical dentists should not wear watches, bracelets and rings;
- After treatment by medical staff, hands should be dried with either disposable paper napkins or clean fabric napkins (sterile napkins are provided for surgeons).
Hand hygiene can be carried out using warm water and soap, or a special antiseptic that reduces the number of microorganisms on the skin of medical staff.
Accounting for costs of compliance with sanitary standards.
Depending on the economic content of contracts concluded with specialized organizations, expenses for compliance with sanitary requirements by medical institutions are reflected according to expense type code 244 “Other purchase of goods, works and services” using:
- Article 340 of KOSGU - when purchasing disinfecting materials, sanitary clothing and footwear;
- subarticles 225 of KOSGU - under contracts for maintenance, warranty and current repairs of sterilizers;
- subarticle 226 of KOSGU - when concluding contracts for advanced training of medical workers conducting endoscopic examinations.
We draw the attention of readers that insufficient funds allocated from the budget cannot serve as a basis for exempting a medical institution from fulfilling the duties assigned to it by law, including taking into account the nature and degree of public danger of an administrative offense. Failure by an institution to comply with SanPiN requirements can lead to negative consequences, contribute to increased morbidity and lead to an unacceptable risk to the life and health of patients (see Resolution of the Armed Forces of the Russian Federation dated February 16, 2015 No. 52-AD15-1).