1620
Barrier membranes are a modern surgical thin-sheet material designed to isolate surgical wounds filled with bone substitute for guided osteoregeneration.
Membranes are used to increase the efficiency of bone regeneration, accelerate wound healing, and eliminate bone resorption and postoperative complications.
Barrier films are used in dental surgery for various types of bone grafting, flap periodontal surgery, surgical correction of maxillodental anomalies, etc.
General overview
The American company Osteogenics Biomedical produces barrier membranes of various characteristics from natural and artificial materials.
Resorbable materials are made from natural raw materials - cattle tendons and porcine collagen.
The starting material for non-resorbable membranes is polytetrafluoroethylene. PTFE material can have a non-textured or textured surface, or be modified (hardened) with titanium.
Osteogenics Biomedical Barrier Membranes
| Resorbable | Size, mm | Non-resorbable | |||||
| Untextured | Size, mm | Microtextured | Size, mm | Titanium Reinforced | Size, mm | ||
| Cytoplast RTM Collagen | 30x40, 20x30, 15x20. | Cytoplast GBR-200 Singles | 12x24 | Cytoplast TXT-200 Singles | 12x24 | Cytoplast TI-250 ANTERIOR | 14x24, 12x24 |
| Cytoplast GBR-200 | 25x30 | Cytoplast TXT-200 | 25x30 | Cytoplast TI-250 BUCCAL | 17x25 | ||
| Cytoplast TI-250 POSTERIOR | 25x30, 20x25 | ||||||
| Cytoplast TI-250 XL | 30x40 | ||||||
Non-resorbable polytetrafluoroethylene (PTFE) dental membrane is an exclusive product not produced by anyone else other than Osteogenics Biomedical.
The material is intended for targeted regeneration of bone and gum tissue during surgical operations in dentistry.
Among the main purposes of Cytoplast GBR-200:
- providing primary wound coverage;
- preventing bone resorption;
- stimulating the growth of bone tissue from osteosubstitute;
- protecting the wound from pathogens;
- acceleration of wound healing;
- simplifying the work of surgeons.
Correction of malocclusions using Vector Tas microimplants and features of their use.
Come here if you are interested in the characteristics and purpose of Bio Oss bone material.
At this address https://www.vash-dentist.ru/implantatsiya/metodiki/temperaturyi-posle-zubov.html find out how long the temperature lasts after dental implantation with a positive outcome of the operation, and what indicators indicate the need to see a doctor.
Resorbable collagen membranes
Dental membranes "Bio Vin" (15x20/20x30/30x40) have good biocompatibility and are made from porcine collagen types I and III.
They can be cut into required shapes, are flexible and perfectly adapt to imperfections. Dental membranes “RTM Collagen” (15x20/20x30/30x40) have good elasticity, made from bovine tendon with type I purity. The multilayer membrane design and unique fiber structure provide high strength.
| Name | vendor code | Price |
| RTM Collagen 15x20mm - resorbable membrane | RTM 1520 | 143 $ |
| RTM Collagen 20x30mm - resorbable membrane | RTM 2030 | 182 $ |
| RTM Collagen 30x40mm - resorbable membrane | RTM 3040 | 259 $ |
Main characteristics
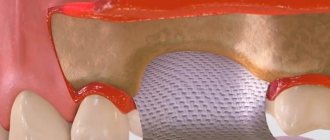
Cytoplast Regentex GBR 200 material is a dense polymer film 0.2 mm thick with a smooth surface.
The latter feature is a significant advantage over traditional resorbable membranes, the surface of which promotes retention and invasion by bacteria, causing rejection of the covering material and inflammation of the wound.
The smooth surface of GBR-200 “repels” pathogenic microorganisms, preventing infection and inflammation of the operating area.
The Cytoplast Regentex membrane has a microporous structure with a pore size of up to 1.26 microns, which does not allow bacteria to pass through the film, but at the same time is permeable to proteins.
The plastic-elastic consistency of the material allows it to be stretched and deformed, giving it the desired shape to adapt to the operating area.
Cytoplast has self-adhesive properties to the wound surface. As an additional measure of fixation, suturing the edges of the flap laid over Cytoplast can be used with light, tension-free sutures that do not affect the membrane itself.
The material comes in standard sizes 12x24 mm and 25x30 mm . From these blanks you can cut pieces of any size required in a specific clinical situation with scissors.
How membranes take root during dental implantation
Collagen membrane overlays reduce the risk of complications due to loss of soft tissue. Non-stitched - due to the healing of soft tissues by secondary intention, the wounds are protected from the general inflammatory process. Both types integrate well into the jaw tissue. The bone under the surface of the barrier plate is regenerated in a manner similar to fetal bone growth.
When using absorbable overlays, the postoperative wound heals without the use of additional medications. Often the healing process of non-resorbable membranes is accompanied by complications, in particular, exposure of the structure. This can lead to infection and inflammation, which means it will have to be removed prematurely.
Often, under the barriers a favorable background is created for the development of microbes, so it is not always possible to achieve the desired result.
Composition and properties
The GBR-200 membrane consists of one PTFE (polytetrafluoroethylene (Teflon)) - a fluorocarbon compound that is resistant to almost any aggressive chemical agents.
Under human body conditions, PTFE is absolutely insoluble in substances and physiological fluids.
Physico-chemical and biological properties:
- chemical stability;
- extremely low allergenicity;
- self-adhesiveness to the wound;
- gas and light tightness;
- impermeability to bacteria;
- permeability to proteins.
Advantages and disadvantages
Cytoplast has a number of clinical and technological advantages compared to traditional suture materials, making it very popular among dental surgeons.
Advantages
- There is no need for primary flap closure of the wound fragment (no need to prepare and stretch the flap material).
- Non-germination of epithelium , due to the high barrier properties of the material.
- Elasticity (the membrane stretches in any direction, adapting to the wound).
- Stability of the structure (does not swell).
- There is no need to cover the membrane with anything from above , the film can remain completely exposed. To completely close the wound area, one Cytoplast GBR-200 is sufficient.
- Self-adhesive to the wound due to its special microstructure. There is no need to use complex fixing elements to attach the film to the bone; the edges of the wound do not need to be sutured.
- The absence of any negative consequences and chemical reactions when the material comes into contact with intraoral fluid.
- Fast wound healing.
- Preservation of the shape of the augmented alveolar bone tissue.
- Easy non-surgical removal of the membrane from the wound in 21-25 days. Removal is done through a small hole using a probe.
- Complete absence of any complications.
Flaws
Numerous tests have not revealed any significant drawbacks, which are often observed when using other types of membranes.
No rejection, inflammation, proliferation or other tissue reactions that negatively affected the healing process were noted.
The only drawback is the need for a dental surgeon to have practical skill in using the material , for which he has to undergo preliminary training.
Types of biomaterials used
The method of directed tissue regeneration in surgical dentistry is based on the use of two main groups of membranes: resorbable (collagen, vicryl, polylactic acid, etc.) and non-resorbable (Gore-tex or titanium mesh, with titanium reinforcement, Teflon and others).
Resorbable
Installation of absorbable membranes prevents their removal after the osteoplastic material has engrafted. Such linings do not always remain stable over a long period. To enhance the prolonged action, their composition is supplemented with slowly resorbing substances. One of the main advantages of membrane linings is the possibility of enriching their structure with drugs that enhance osteogenesis, anti-inflammatory, antimicrobial agents, etc.
Types of resorbable membranes:
- Synthetic polymers (polyactides, polyglycolides) and their chemical modifications (for example, Guidor, EpiGuide, Vicryl) - decomposition into microscopic fragments that undergo phagocytosis occurs under the influence of hydrolysis. Among the disadvantages of the products, weak tissue integration and a high likelihood of developing swelling during resorption due to changes in pH in the tissues are noted. Advantages of polymer plates:
- no risk of transmitting an infectious disease;
- hypoallergenic;
- long-term effect (5-6 months).
Non-resorbable
They contribute to the effective restoration of bone in a given volume and trajectory, there are:
- Frameless - for restoration of bone tissue less than 4 mm.
- With a titanium frame - for bone restoration in large volumes, in all directions.
Main advantages:
- high mechanical strength;
- pronounced barrier properties.
These types of pads should be removed after 6-9 months . Consisting of titanium, they are effective in situations where there is a large load on the bone and it is necessary to protect implants or osteoplastic material. The most common indication for use is vertical ridge augmentation. They are removed after restoration of soft and bone tissues damaged during the operation.
Main disadvantages:
- risk of exposure - during suturing of the flap, the insert must be completely closed;
- absence of the process of periosteum formation under the overlay;
- the patient needs to visit the dentist frequently (every 2 weeks).
Statistics show that the most effective membranes are polytetrafluoroethylene (PTFE) GoreTex and aliphatic polyetherurethane (Bone-up). Less commonly used are mesh forms made of calcium sulfate (Capset) or titanium (Frios, BoneShield, Tiomesh).
Indications
The GBR-200 membrane is recommended for use in dentistry in all cases associated with directed bone regeneration:
- After tooth extraction to restore the bone tissue of the alveolar process.
- Before installing an implant if the bone volume is small.
- During or after installation of the implant - to close the wound.
- For augmentation in a wide variety of clinical cases.
- For peri-implantitis (inflammation of bone and soft tissues adjacent to the implant, their resorption and loss of the implant).
- For flap operations in the oral cavity, plastic surgery and gum recession.
- When restoring jaw proportions (for example, with a planar osteotomy, accompanied by replanting of bone tissue between separated fragments of the jaw bones).
- During sinus lifting (Cytoplast is placed on the bottom of the maxillary sinus to eliminate the risk of penetration of the osteosubstitute into the sinus through perforations in the mucous membrane).
Let's discuss together the pros and cons of dental implantation in comparison with other prosthetic methods.
In this publication we will tell you whether you need to take antibiotics after dental implantation and why.
Follow the link https://www.vash-dentist.ru/implantatsiya/metodiki/rashozhdeniya-shvov-posle-zubov.html to find out when the sutures are removed after dental implantation.
What are barrier membranes?
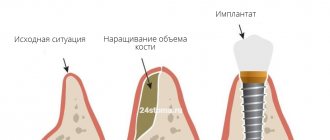
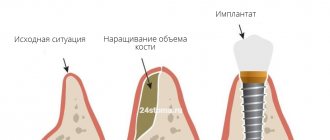
The barrier membrane is one of the most modern and successful inventions in dental practice. Such designs are actively used in almost all surgical interventions. Barrier membranes for bone grafting during implantation can prevent atrophic phenomena in the alveolar process and contribute to the immediate successful fixation of artificial roots. The membrane affects the regenerative abilities of the bone, increasing them, does not allow osteoclasts that are dangerous to bone cells to enter, and minimizes the risk of penetration of pathogenic agents. Thanks to the barrier membrane, graft engraftment is accelerated, as is the overall treatment of the patient.
The membrane for bone grafting is a very thin and fairly elastic plate that is attached to the bone with titanium pins. In this way, the gums are separated from the bone material during the formation of natural jaw tissue.
Types of membranes
There are two types of barrier membranes that are widely used in dentistry:
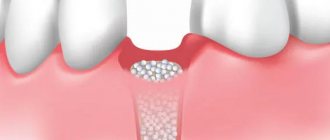
- Resorbable. This structure gradually dissolves. It does not require separate removal, that is, additional surgical intervention, which means it causes less trauma.
- Non-resorbable. Membranes of this type do not dissolve on their own, which is why patients need an additional stage of treatment - removal of the structure. In most cases they are made of titanium. Such membranes are attached in the presence of a serious deficiency of bone tissue, filled with granules of osteogenic material. Once the alveolar bone and soft gum tissue have recovered, the barrier membrane is removed.
The dentist independently selects the optimal membrane for a specific clinical case. He must take into account that resorbable structures do not fix the grafts well enough, so they are used only for minor atrophies.
Technique of use
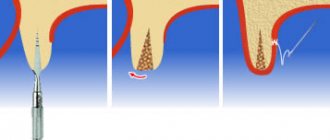
After filling the surgical wound with a bone substitute, Cytoplast GBR-200 is placed on top of it, overlapping the edges of the wound by 2-3 mm. In addition to self-adhesiveness, the film can be fixed to the wound using light, tension-free suturing of flaps placed on top of the Cytoplast.
The membrane is removed 21-25 days after the operation. The deadline is 28 days. Repeated surgery to remove Cytoplast is not required.
If a flap has been placed and sutured over the membrane, the material is extracted (pulled out) using a probe through a small hole . In most cases, anesthesia is not required (in extreme cases, topical anesthesia is used), bleeding, as a rule, does not occur.
After covering the surgical wound under the Cytoplast, bone and secondary epithelium begin to quickly form. Fusion of the flap with the newly formed epithelium usually occurs a month and a half after the operation.
In the video, watch the technique of using the Cytoplast membrane.
Membrane exposure after bone grafting - consequences and treatment
The non-resorbable membrane pad is exposed in 1% of cases , causing the following consequences:
- wound infection with the development of purulent inflammation;
- rejection of osteoplastic material during vascularization of the mucous membrane (in 40-50% of cases).
In such situations, it is recommended to remove the implanted materials, carry out treatment, and then repeat bone grafting. Typically, a second layer of soft tissue is formed under the incision using a mucoperiosteal flap. To do this, it is peeled off, split, modeled, and then sutured in layers.
The exposed resorbable pad should be treated with 3% hydrogen peroxide and 0.12% chlorhexidine. Sometimes this allows you to postpone the removal procedure by 8 weeks.
Opinion of a dental surgeon : “Most of my patients are smokers. Many people do not immediately realize the complexity of the situation caused by their bad habit. Smoking causes a sharp narrowing of the blood vessels in the mouth. This weakens the flow of blood filled with oxygen and nutrients. Due to their lack, the healing process is delayed. Therefore, you need to completely stop smoking at least a couple of weeks before implantation and for several months after it.”
Clinical results
In order to determine the effectiveness of the GBR-200 membrane, the developer conducted studies of the material on a group of 10 patients in 3 clinical cases:
- tooth extraction;
- tooth extraction with implantation of a bone substitute;
- tooth extraction with implantation.
The following conditions for the operation were provided:
- the membrane was cut out using scissors according to the shape of the defect;
- when applied, the membrane overlapped the hole by 2-3 mm;
- adherence of the material to the wound was ensured by stretching and pressing with fingers and an instrument, taking into account the configuration of the alveolar process;
- The cytoplast remained on the wound for 21 days, after which it was carefully pulled off with tweezers.
Research results
- In no case of use of the GBR-200 material did an inflammatory process, cell proliferation or other negative tissue reactions occur.
- None of the membranes were rejected.
- All films were easily removed from the wound after 21 days without the use of anesthesia.
- On the wound surface, which was under the membrane, there was no resorption of surrounding tissues or epithelial growth. Only in some places along the edges of the wound were there migrating small areas of epithelium.
- The wound surface after removal of the Cytoplast was a newly formed bone structure.
Based on the results of the study, it was concluded that when Cytoplast GBR-200 was used for 3 weeks, a reliable barrier was created on the surface of the wound, which prevented the germination of epithelial tissue.
Guided bone regeneration (GBR) is used to place titanium dental implants.
The use of a membrane to prevent non-osteogenic tissue from interfering with bone regeneration is a key principle of the procedure. Today, numerous types of dental membranes are used in experiments and clinical practice. This requires up-to-date information on material properties and biological outcomes, as well as a critical assessment of the biological mechanisms governing bone regeneration in membrane-covered defects.
Experimental data indicate that various modifications of the physicochemical or mechanical properties of membranes can promote bone tissue regeneration. However, the role of porosity on the barrier function of these products still needs to be clarified.
New experimental data indicate the active role of the membrane compartment in stimulating regenerative processes. Optimizing materials by systematically considering barrier or biological properties is an important strategy in this area of research.
Basics of Guided Bone Regeneration
New methods of rehabilitation for edentulism using osseointegrated implants have caused a revolution in dentistry and significantly improved the quality of life of patients.
However, bone loss or insufficiency, as a symptom of a variety of systemic and periodontal diseases, trauma and tumors, remains a major challenge to osseointegration.
To achieve an adequate long-term prognosis for the installation of osseointegrated implants, the proposed implantation site must contain a sufficient amount of healthy bone tissue.
Various strategies such as bone grafting techniques, alveolar distraction, and guided bone regeneration (GBR) are used to restore lost bone, allowing for complete implant integration and support during functional loading.
Guided regeneration is considered one of the most important methods most commonly used to restore alveolar bone and treat peri-implant bone deficiency.
NRC, using barrier membranes to exclude certain cells such as rapidly proliferating epithelium and connective tissue, promotes the growth of slow-growing cells capable of bone formation. The method is often combined with bone grafting procedures.
Guided bone regeneration is achieved when osteoprogenitor cells are given the opportunity to populate the site of a bone defect, preventing the invasion of non-osteogenic tissue.
According to foreign researchers, about 40% of osseointegrated implants will require NDT as part of a standard dental rehabilitation plan.
Several studies have indicated that implant survival in sites augmented with NRC is similar to that reported for implants in control sites.
Implant survival rates ranged from 79% to 100%, with most clinical studies reporting survival rates greater than 90% after at least 1 year of function.
Dental membranes are the most important material for the procedure. For the production of these products, various natural and synthetic materials and their modifications have been proposed:
- Synthetic membranes made of polytetrafluoroethylene: a polymer that is inert and stable in biological systems, but does not undergo natural resorption after implementation.
- Synthetic membranes made of aliphatic polyesters (PLA, PGA, PCL): characterized by bioresorbability, good processability and controllability, can encapsulate drugs, but do not have sufficient rigidity and strength.
- Natural membranes made from collagen from cows, pigs and other animals: characterized by bioresorbability, low immunogenicity and the ability to encapsulate drugs.
- Dental membranes made of titanium and cobalt and chromium alloys: have sufficient mechanical strength and ductility, but are not resorbable.
- Inorganic membranes made of calcium phosphate (hydroxyapatite): have pronounced osteoconductive properties, but their strength and plasticity are insufficient.
For the success of guided bone regeneration, a material has a number of important requirements, including biocompatibility, cell occlusion, complete integration with host tissues, clinical controllability, volume-generating ability, and adequate mechanical and physical properties.
Non-resorbable membranes, mainly consisting of polytetrafluoroethylene (PTFE) in its modified form (e-PTFE), constituted the first generation of barrier membranes.
In general, these product types demonstrate biocompatibility and volume-creating ability. However, non-resorbable membranes require new surgical intervention to remove the barrier.
Subsequently, a second generation of membranes made of absorbable (resorbable) materials was developed and became widely used in various clinical situations. Recently, efforts have been made to develop a new generation of products that actively use naturally occurring materials and tissue engineering principles in the manufacturing process.
In addition, the use of membranes in the defect together with bone grafts or bone replacement materials is now favored to provide structural support to the defect site and to promote the intrinsic regenerative potential of the host tissue.
Below, we present a comprehensive review of attempts to modify membrane properties and associated effects in light of the biological mechanisms regulating bone regeneration in NSC.
Clinical applications of guided bone regeneration
Although this review does not focus on the clinical outcomes of different treatments for NCC, there is a need for a summary of the main clinical indications.
Alveolar bone resorption compromises the structural, functional, and esthetic results of a dental implant procedure. After tooth loss, alveolar bone resorption occurs first in the horizontal direction, during the first 6 months, and then in the vertical direction.
There are several strategies to increase alveolar bone volume, including NBR, distraction osteogenesis, ridge splitting, free vascularized autografts, or maxillary sinus floor elevation.
The severity of bone loss and the configuration of bone defects determine the type, extent, and prognosis of treatment. Although clinical data show high survival rates for implants placed in augmented bone, some techniques do not yet have reliable evidence of long-term clinical effectiveness.
It is generally accepted that increasing bone volume remains a problem in vertical bone defects and progressive horizontal atrophy of the alveolar processes.
Guided bone regeneration is a successful, well-documented and widely used procedure for the treatment of alveolar bone defects in combination with dental implantation. Recent reviews have shown a 95% survival rate for implants following either a horizontal or vertical procedure.
Currently, NBR involves the use of various types of membranes (resorbable or non-resorbable) in combination with various bone replacement materials. The choice of materials depends largely on the size and configuration of the bone defect.
Clinical studies show that NBR is predictable and successful in horizontal defect augmentation, and in most cases success can be achieved with non-resorbable or resorbable dental membranes.
Resorbable membranes are considered more convenient to use. Although excellent results have been obtained with nonresorbable membranes, several publications indicate that such membranes are subject to a higher complication rate.
Complications are mainly associated with prolonged exposure to soft tissue. A plausible explanation for the complications is tension in the soft tissues combined with insufficient vascular supply.
However, the exact biological mechanisms of membrane action are not yet fully understood. When exposed to a resorbable membrane, spontaneous healing was often observed, possibly resulting from rapid membrane degradation rather than soft tissue proliferation.
Although horizontal augmentation had a more predictable outcome than vertical augmentation, many reports have demonstrated the beneficial effects of NRC using nonresorbable e-PTFE membranes.
Clinical studies have also used a titanium-reinforced e-PTFE membrane in combination with bone replacement materials to improve the results of vertical augmentation.
Although nonresorbable membranes have been more commonly used for vertical bone defects, recent clinical studies using collagen-based resorbable membranes have shown promising results.
As previously mentioned, the main complication associated with nonresorbable membranes is soft tissue exposure. The complication is more common with vertical bone augmentation, in which the lack of soft tissue is clinically considered a limiting factor.
To improve outcome, especially in complex clinical cases, bioactive regenerative approaches, such as the use of recombinant growth factors in combination with NRC, have been discussed.
Studies have shown that the addition of recombinant human platelet-derived growth factor-BB (rhPDGF-BB) with bone grafting material under a resorbable membrane has a positive effect on soft tissue healing and provides better bone preservation after 1 year of implant loading.
However, there is no convincing clinical evidence for the beneficial effects of growth factors, and development in this area remains somewhat limited due to legal regulation in some countries around the world.
Platelet concentrates, including platelet-rich plasma and platelet-rich fibrin, have been proposed as additional stimuli for bone regeneration.
Initially, platelet concentrates were used as autologous scaffolds for NRC or other applications in oral and maxillofacial surgery. Platelet concentrates are obtained from the patient's blood; these drugs contain platelets and white blood cells with the potential to secrete various growth factors and cytokines, thereby accelerating wound healing.
It has been suggested that platelet-rich fibrin may act as a bioactive membrane for NRC, but only a few clinical reports on this topic have been published.
Mechanical properties and degradation of such membranes can be a problem.
Kawase and colleagues were able to reduce the biodegradation rate of platelet-rich fibrin membrane using a thermal compression technique that did not sacrifice biocompatibility. But until now, the use of platelet-rich fibrin membranes has been less documented for NRC than for GTR.
Taken together, clinical trials, meta-analyses, and systematic reviews demonstrate successful results with NRC procedures for alveolar bone augmentation and implant placement.
However, some clinical situations remain controversial, especially in cases of vertical and progressive horizontal alveolar atrophy.
Most studies used non-resorbable e-PTFE membranes or resorbable collagen-based membranes. The evolution of these products is primarily driven by the required barrier function, patient convenience, and ease of handling in various clinical situations.
Selection of Dental Membranes for Guided Bone Regeneration
From a chemical point of view, dental membranes for NRC can be classified as synthetic polymers, natural polymers, metals and inorganic compounds.
Synthetic polymers
The first registered synthetic polymer for NRC was e-PTFE.
It is considered one of the most inert and stable polymers in a biological system. It resists degradation by host tissues and does not cause significant immunological reactions.
The chemical stability of e-PTFE maintains structural integrity and insulating function. But oral exposure to e-PTFE leads to microbial migration and bacterial infection, which may compromise bone growth and osseointegration.
Aliphatic polyesters
Another widely used category of synthetic polymers are polylactic acid (PLA), polyglycolic acid (PGA), poly(ε)-caprolactone (PCL), polyhydroxyvaleric acid, polyhydroxybutyric acid, and their copolymers.
The main advantages of these types of polymer dental membranes are their controllability, manufacturability, optimal biodegradation and the ability to encapsulate drugs.
However, their degradation can cause a strong inflammatory response leading to resorption of regenerated bone. Their lack of rigidity and stability can in some cases be considered as major disadvantages.
The high rate of degradation of aliphatic polyesters reduces the barrier membrane's operational time and space-creating capacity, which affects the outcome of bone regeneration.
However, clinical studies have demonstrated the possibility of successfully using a polyester membrane to preserve and augment alveolar bone after dentition.
In fact, the rate of resorption of these products depends largely on the type of polymer.
For example, PCL is characterized by higher hydrophobicity and lower water solubility than similar PLA and PGA membranes. Copolymer-based products (eg, lactide, ε-caprolactone, glycolide, and trimethylene carbonate) have been proposed to reduce the rate of resorption.
Natural polymers
Collagen-based products are the most commonly used natural membranes for targeted bone regeneration. They receive great attention because collagen is a major component of connective tissue, provides adequate structural support and is an indispensable component in intercellular communication.
Collagen membranes
has many clinical applications that make the material interesting for many fields. Although comparable clinical results are known between collagen and non-resorbable membranes, other studies have shown that collagen products may promote even better wound healing and bone regeneration.
The most important disadvantage of collagen membranes is their lack of rigidity. Therefore, their use is more acceptable in alveolar ridge defects such as dehiscence and fenestration, which do not require additional fixation for stability.
There is currently a wide range of these products available in the market.
Collagen membranes are produced from bovine and porcine tissues (eg, tendon, dermis and small intestine), and their biodegradation varies depending on the source of origin. The rate of degradation of the collagen membrane may not correspond to optimal tissue regeneration.
A number of specific physical/chemical cross-linking technologies have been used to optimize the mechanical properties of the collagen membrane and slow down the rate of degradation. These methods include ultraviolet (UV) radiation and treatment with chemical solutions such as genipin (Gp), glutaraldehyde, and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC).
Although chemical cross-linking has resulted in improved collagen stability, chemical residues (eg, amides or aldehydes) can cause severe inflammation at the implantation site.
Thus, the predictability of a collagen membrane depends not only on the origin of the collagen material, but also on the preparation and processing procedures (decellularization, sterilization, and cross-linking).
Natural compounds such as Gp and D-ribose have been proposed as safe, non-toxic, non-immunogenic agents to provide high mechanical strength and low degradation rates.
Collagen-based membranes are also obtained from the human body. Acellular dermal matrix (ADM) is produced from human skin after removal of the epidermis and all skin cells.
The collagen and elastin structure of the extracellular matrix (ECM) as well as endogenous growth factors have been shown to be retained in ADM after decellularization. Biomechanical analysis showed that ADM has better strength and stiffness than cellular dermal membrane.
Abroad, ADM has been clinically used for the preservation of alveolar processes and the treatment of peri-implantation defects.
Other types of collagen membranes are derived from human pericardium and dura mater.
There have also been attempts to use human amnion structures to make biocompatible membranes using decellularization and sterilization techniques. Lyophilized multilayer amniotic membrane retains the structural and mechanical properties of the amnion ECM and has sufficient flexibility in adjusting thickness and mechanical properties.
It is assumed that this membrane promotes intensive growth of bone tissue, while simultaneously limiting the penetration of fibrous tissue, that is, performing an isolating function.
Chitosan
is another natural polymer used to prepare NRC membranes. This material consists of copolymers of glucosamine and N-acetylglucosamine. It can be produced by partial deacetylation of chitin.
The latter material is present in the shells of crustaceans (shrimp and crabs), where it plays a role similar to that of collagen in higher animals. Chitosan has essential properties including biocompatibility, biodegradability, low immunogenicity and bacteriostatic effect.
The rate of degradation of chitosan membranes depends on the molecular weight, as well as on the production technology. Like collagen, chitosan can be cross-linked using glutaraldehyde and Gp.
However, due to the toxicity of glutaraldehyde and the high cost of Gp, the use of sodium tripolyphosphate has been proposed as an alternative cross-linking method.
Alginate membranes
have also been used for guided bone regeneration.
Alginate is a biocompatible anionic polymer typically derived from brown algae. When cross-linked with hydrogels, it has a structure similar to the extracellular matrix.
Although there is evidence that both chitosan- and alginate-based membranes promote bone regeneration in experimental bone defects and are suitable materials, reliable clinical evidence supporting these materials has not been published worldwide.
Titanium and other metals
Titanium is a widely used material in dentistry, craniofacial surgery and orthopedics.
Its advantages include biocompatibility, high strength and rigidity, low density and weight, ability to withstand high temperatures and corrosion resistance. The use of titanium for NBR was inspired by the excellent results of titanium mesh in the reconstruction of maxillofacial defects.
Many foreign studies have shown that the use of titanium mesh alone and in combination with bone substitutes is an effective option for local alveolar bone augmentation before or simultaneously with the installation of a dental implant.
Occlusive titanium and microperforated titanium membrane have also been used for the treatment of peri-implant defects and alveolar bone augmentation. In several studies, scientists have compared the biocompatibility of titanium with other membrane materials.
There is experimental evidence that titanium causes less persistent inflammation than PTFE.
Alloy based on cobalt and chromium
(CoCr) has also been proposed for NDC. Although this alloy is known to be less biocompatible than titanium or titanium alloy, it has excellent mechanical properties (such as stiffness and toughness).
The potential of using CoCr alloy for guided bone regeneration has been evaluated in recent animal studies, but has not yet been documented in any clinical report.
Decco et al report that placement of a CoCr membrane in a rabbit tibial defect provides sufficient volume and promotes bone regeneration.
Inorganic materials
Calcium sulfate is one of the few inorganic compounds that have been used for mass production of membranes. It is a biocompatible, osteoconductive and bioresorbable material. The material occurs naturally and can be produced synthetically.
Calcium sulfate membranes are produced by hydrating hemihydrate powder (gypsum of Paris). The latter produces a paste that can be molded and injected into a rigid material with relatively stable, less resorbable crystals.
Membrane based on hydroxyapatite
(GAP) is also used for NDT. Hydroxyapatite is a calcium phosphate material widely used in osteoplasty due to its similarity to natural bone mineral, biocompatibility and osteoconductivity.
HAP is less resorbable than many other calcium phosphate materials. Although HAP is considered relatively fragile, it exhibits adequate mechanical properties, allowing the membrane to withstand static pressure from soft tissues and maintaining more space for bone regeneration.
Hydroxyapatite dental membranes have been shown to promote high functional activity of stromal and osteoblast-like cells in vitro and induce bone formation in vivo.
HAP powder used to prepare pure ceramic membrane or other types of products is also combined with bioactive ions including strontium, silver and zinc to enhance their in vivo biological effectiveness.
Other ceramic materials such as beta-tricalcium phosphate
(β-TCP), have been incorporated into resorbable membranes and have demonstrated pro-osteogenic effects in vitro and in vivo.
Moreover, the addition of bioactive glass nanoparticles to bioresorbable membranes increases the metabolic activity and mineralization of cells in vitro.
When using a collagen membrane with bioactive glass, increased bone regeneration was demonstrated compared to native collagen membrane.
Properties of dental membranes
For the success of targeted bone regeneration and subsequent implantation, dental membranes must have certain properties. Let us list the most important of them.
Rigidity and ductility
The amount of regenerated bone in a bone defect will be reduced if the membrane is destroyed.
Therefore, the ideal material should be stiff enough to withstand the compression of the overlying soft tissue. At the same time, it must have a certain degree of plasticity so that it can be easily contoured, giving the shape of the defect.
A balance between these mechanical characteristics is necessary to achieve adequate volume of space. Titanium has excellent mechanical properties compared to other types of materials such as bovine collagen or e-PTFE.
While rigidity prevents membrane failure and maintains volume, its ductility allows the material to bend and adapt to the defect. But the cut edges of titanium mesh sometimes cause irritation of the mucous membrane, leading to membrane exposure and infection.
In a rabbit study, placement of a titanium membrane over a maxillary defect caused a higher rate of bone regeneration compared to a PTFE product (Lundgren et al., 1998). This was mainly due to the preservation of the volume of the titanium membrane.
To increase the rigidity of the PTFE membrane, a titanium frame is integrated into the membrane structure, which provides additional stability of the membrane during treatment. This modification also made it possible to smoothly change the shape of the membrane in accordance with the characteristics of the case.
On the other hand, due to insufficient rigidity, especially in the case of resorbable membranes, the bone defect is often filled with bone replacement material to prevent fracture and preserve volume.
Miniscrews and pins have also been used to stabilize the membrane in the surrounding bone to reduce the risk of collapse. In addition, a technique using a tent screw has been proposed to provide and maintain the required space during ridge augmentation.
Calcium phosphates are included in resorbable membranes to improve mechanical properties. For example, the incorporation of β-TCP into a polymer product made from PCL/PLGA can increase mechanical stability and promote bone regeneration in vivo.
It has also been shown that the mechanical properties of collagen and polyvinyl matrix are significantly improved after the addition of β-TCP/chitosan composite and nano-hydroxyapatite, respectively.
In addition, the composite product based on HAp and amino acid copolymer with low nanocalcium content demonstrated adequate biomechanical properties. Interestingly, collagen-based trilayer membranes have been developed to optimize mechanical properties.
While the top and bottom layers of the membrane are composed of hydroxyapatite-containing collagen for better flexibility and biological activity, the middle layer is composed of chitosan to provide high strength and increase the elasticity of the entire structure.
It has been shown that the compressive strength of a PLLA membrane can be controlled by changing the molecular weight of the polymer.
Compared to the 100,000 MW PLLA membrane, the 380,000 MW PLLA membrane exhibits higher compressive strength equal to that of titanium mesh and is considered a good choice for vertical augmentation.
Porosity of material
Porosity is an important property of a dental membrane.
Studies published to date have addressed the role of porosity in inducing biological responses in vivo using resorbable and nonresorbable membranes.
The size of the pores influences the degree of bone regeneration in the underlying isolated space.
It is believed that this indicator is closely related to tissue occlusion and has a significant impact on the penetration of soft tissue cells. Pores have also been reported to facilitate the diffusion of fluids, oxygen, nutrients and bioactive substances for cell growth.
However, the presence of too large pores can impair occlusal properties by allowing soft tissue cells to migrate through the membrane. They overpopulate the defect site, suppressing the infiltration and activity of osteoprogenitor cells.
The presence of 5-30 µm pores in the e-PTFE membrane has been reported to promote bacterial contamination and strong soft tissue attachment. Therefore, dense d-PTFE was developed with submicron pores (0.2 μm) that prevent the migration of bacteria into the structure.
Higher adhesion of Actinobacillus actinomycetemcomitans, Treponema denticola and Porphyromonas gingivalis was found on collagen membranes than on e-PTFE and d-PTFE, but no differences in bacterial adhesion of microorganisms were found between PTFE.
Several studies have indicated that the use of d-PTFE prevents the entry of microorganisms, inhibits infection at the regeneration site, and does not even require primary closure.
Lower porosity made PTFE membranes less prone to soft tissue attachment and thus can be easily removed without the need for additional surgical procedures.
However, minimal tissue integration with d-PTFE membranes poses potential problems for initial clot formation, wound stabilization, and membrane stability.
Placement of e-PTFE containing 300 μm pores in combination with titanium implants has been shown to provide sufficient space and significant vertical bone gain.
At both the microscopic and macroscopic scales, Lundgren and colleagues studied the effect of pore size on NRC in rats using a rigid plastic plate as a solid or occlusive membrane and six polyester meshes of varying porosity (10, 25, 50, 75, 100 and 300 µm).
The low rate of bone gain was associated with the occlusal barrier. In contrast, placement of polyester meshes with perforations greater than 10 µm results in a higher rate of bone growth than when using meshes with pores of 10 µm.
These and other experimental data show that more porous membranes (20-25 or 100 µm) cause faster and more efficient regeneration compared to finely porous materials.
Moreover, according to data provided by Gutta and colleagues, macropores in titanium membrane with a diameter greater than 1 mm promote better bone regeneration.
This latter observation is supported by the fact that although titanium mesh has a macroporous structure and presumably allows migration of non-osteogenic soft tissue to the defect site, it still remains one of the most predictable options for horizontal and vertical augmentation.
Although it has been suggested that the less porous polylactide membrane preserves osteogenic components in the defect site, some authors have argued that the presence of large holes (800–900 μm) provides adequate vascularization for the treatment of large bone defects.
In reality, the pore size and degree of porosity of the available membranes vary, and the optimal membrane porosity has not yet been determined.
Further systematic research is needed to address the following questions. First, does a dental membrane for NDT really need to be porous? Second, what is the role of porosity and permeability in the bone healing mechanism?
Membrane architecture and thickness
Collagen membranes have different structures and thicknesses depending on the source of collagen, the extraction method and the method of manufacturing the product. They may consist of a homogeneous collagen matrix or a bilayer structure.
Ultrastructural evaluation showed that the Jason membrane consists of collagen fibers of various orientations, forming a comb-like structure with strong multidirectional bonding. DynaMatrix has discrete layers of collagen hard shells. Collprotect is considered semi-permeable due to its open porous and three-dimensional structure.
Dual-layer membranes such as BioGide and Mucograft have a single compact layer that is capable of preventing epithelial cells from penetrating into the bone defect. The second, porous spongy layer of the structure is responsible for tissue integration. This structure was simulated for a synthetic commercial glycolide-trimethyline carbonate copolymer membrane (Resolut).
In another two-layer polymer membrane, Guidor, the two layers are made in a grid pattern, but with different pore sizes and geometries. While the outer layer had large, rectangular-shaped pores to allow integration of the overlying soft tissue and promote tissue integration, the inner layer had relatively small, circular-shaped pores to limit tissue permeability.
In fact, the design and architecture of polymer membranes are the most important factors for bioresorbability and osteopromoting effect in vivo.
The membranes described above differ not only in their architecture but also in thickness, which may influence their mechanical and space-saving properties upon implantation.
Placing a thicker collagen membrane is known to result in less soft tissue ingrowth and promote better bone formation.
Finally, assembling two layers of the same type of non-crosslinked collagen membrane reduces bone graft resorption and improves bone regeneration, as well as retaining the body of the membrane for a longer period of time.
Taken together, data published by different authors indicate an active role of the membrane in stimulating regenerative processes in NCC. This is more than a passive barrier.
On the other hand, it is not yet known whether different membranes will have different potential for mobilization and activation of membrane-recruited cells, and whether this will lead to different results in bone formation and bone defect regeneration.
It is important to obtain such information to make fully informed decisions in clinical practice and to develop the next generation of improved dental membranes.
Reviews
The use of barrier membranes during surgical interventions is a new, modern technology that has not yet received the distribution it deserves.
If you have undergone surgery using Cytoplast barrier membranes, share your impressions of this material with other visitors to our site, leave your comment at the bottom of the page.
If you find an error, please select a piece of text and press Ctrl+Enter.
Tags: implantation, implantation methods
Did you like the article? stay tuned
Previous article
Should I save or remove a tooth in case of purulent periodontitis?
Next article
Universal trainer I-3 for the correction of malocclusions in children
Diagnostics
To identify epiretinal fibrosis, it is necessary to examine the fundus, for which ophthalmoscopy is prescribed. Examination in transmitted light reveals its glimmer over the area of the yellow spot, reminiscent of reflection from cellophane film.
The epiretinal membrane in the initial stage is very thin, it may not be noticed, which complicates diagnosis. However, if there is a suspicion of its presence, an ultrasound examination is prescribed to confirm the diagnosis. It is also necessary when the optical media (vitreous body, lens, cornea) are opaque, which makes it difficult to examine the fundus using ophthalmoscopy.
Another diagnostic method for epiretinal fibrosis is optical coherence tomography, which helps to determine the size and structure of the membrane.
The degree of damage caused by the membrane can be determined by fluorescein angiography, which shows the extent of macular edema. How much the patient’s vision has deteriorated is determined using the Amsler grid test and visometry.
Fig. 2 Vitreoretinal surgeon in the operating room performing vitrectomy and macular peeling.