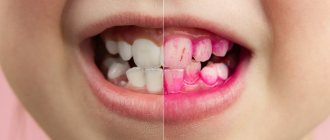
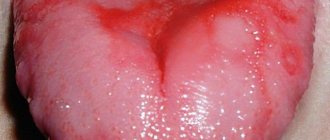
Plaque on the tongue itself is not a disease, it is a symptom that reflects what is happening in the body. There are types of plaque on the tongue that indicate a disorder in the functioning of internal organs. These types of plaque are called pathological.
Author:
- Sadykhov Rahim Agalarovich
ENT pathology expert
3.64 (Votes: 14)
Plaque on the tongue itself is not a disease, it is a symptom that reflects what is happening in the body. In this regard, the statement that language is a mirror of our health is quite true. A slight transparent or whitish coating is normal for a healthy adult. It is usually noticeable after sleep and can be easily cleaned with a toothbrush. However, there are other types of plaque that are not so harmless and indicate a disorder in the functioning of internal organs. These are pathological types.
Pathological plaque can be different: the color ranges from thick white to black, dense and sticky in consistency, and has a bad odor. The structure of the language itself may also change. This is the first signal about the onset of the disease, so if you notice uncharacteristic changes in the appearance of your tongue, consult a doctor for a correct diagnosis. Indeed, in this case it is necessary to identify the root cause of this unpleasant manifestation and quickly begin to treat the underlying disease.
Norm and pathology
Language is a kind of mirror of the functioning of the body. When it is clean, pink, with visible taste buds, the person is healthy and most likely does not suffer from bad habits. But even a loose white coating, through which the surface of the tongue is visible, is considered as a variant of the norm. It can change shade depending on the time of year: in summer it can be more intense due to hot weather, in winter it becomes white-yellow, in autumn it becomes almost transparent. In addition, a yellow coating on the tongue in the morning appears in heavy smokers and those who drink too much coffee.
However, a change in the color of the tongue may be a consequence of malfunctions in the internal organs, mainly the liver, kidneys, stomach, pancreas and gall bladder. If the plaque has become dense, thick, bright yellow or brownish-yellow, and there is also bad breath, you should consult a doctor and identify the cause of the problem.
Attention!
Typically, “safe” soft deposits come off easily and, if brushed well, will not reappear. But if the plaque returns, we are dealing with pathology.
Can smoking cause tongue cancer?
When smoking, the tongue is the first organ to take the blow in the form of hot tobacco smoke, which contains many toxic substances.
With such regular and frequent attacks, the oral cavity and tongue receive injuries in the form of burns .
As a result, small nodules appear , as well as other neoplasms, which over time have the ability to degenerate into cancer.
Primary and secondary signs
You should know! Cancer of the uvula is poorly diagnosed, since clear symptoms of the disease appear only in the later stages.
It is worth noting that this type of cancer develops quickly and causes many serious complications.
Symptoms of the initial stage of the disease are almost invisible.
The insidiousness of the disease lies in the fact that there are no obvious signs until the late stages of the disease begin .
Usually it all starts with the appearance of an ulcer , compaction or nodules on the tongue .
At the early stage of a malignant neoplasm, the boundaries of the nodules that appear on the tongue are clearly defined.
Later, as the disease progresses, the boundaries of such formations become more blurred, the tissue under the ulcer thickens , and its edges rise and resemble a roller in appearance.
Later, pain, swelling of the face, and difficulty opening the mouth and eating appear.
Classification of cancer of this organ by localization:
Uvula body cancer
This type of cancer is the most common .
Know! A malignant tumor affects the middle part and can often affect its lateral areas.
Tongue root cancer
If the root of this organ is affected when swallowing, severe pain is felt in its area .
This type of cancer is less common than its previous type.
The disease also has another name - oropharyngeal cancer , in which the tumor is located in the posterior parts of the oral cavity.
The pathology has an aggressive course and is difficult to treat.
Cancer of the lower part of the tongue
With this pathology, the tumor is located on the lower part of the uvula. In rare cases, it may be located under the tongue.
Note! Histological differentiation of cancer types:
- Adenocarcinoma;
- Squamous cell carcinoma.
There are three main types of tongue cancer tumors :
- Ulcerative. This form begins with a lump that appears on the tongue, which soon becomes an ulcer. The patient experiences pain and the ulcer bleeds.
- Infiltrative. The new growth that appears is hard to the touch. Its surface is covered with whitish spots. The patient experiences severe pain in this area.
- Papillary. The neoplasm is hard, has dense plaques and protrudes above the surrounding healthy tissue.
Secondary symptoms
- layer thickness. If the natural color of the tongue is not visible through the plaque, we are dealing with severe processes or chronic diseases. A thin layer, on the contrary, indicates an initial stage or a slight deviation from the norm;
- coverage area (fully or partially);
- consistency. It can be thick, soft, dry or flaky;
- ease of removal. Dense thick plaque is usually difficult to remove. A softer, thinner layer is easier to clean, but can quickly reappear.
Abstract | Cigarette smoking is inextricably linked with serious pathology of all systems of the human body, and the skin is no exception. The subject of this review is the harm that tobacco consumption causes to the skin. It is important to determine the difference between the effects of smoking and the effects of nicotine on the skin. All skin cells express several subtypes of nicotinic acetylcholine receptors (nAChRs), including the α7 receptor subtype. Smoking affects some chronic dermatoses negatively, while others have a positive effect. Having identified the negative impact of smoking on a certain disease, patients can be advised to quit it, and in the opposite cases, therapeutic agents based on nAChR agonists can be developed. Toxic components of tobacco products Cigarette smoking is inextricably linked with serious pathology of all systems of the human body, and the skin is no exception. Despite attempts to warn the population about the dangers of smoking, it remains a leading cause of preventable death. Tobacco smoking has been known in Europe since Christopher Columbus noticed the Arawak Indians smoking tobacco leaves in 1492. The tobacco plant was named Nicotiana tabacum in honor of the French ambassador to Portugal, who introduced France to this plant - Jean Nicot. Nicotine, the main alkaloid in tobacco, was isolated in 1828. Nicotine can enter the human body through smoke inhalation into the lungs or gastrointestinal tract, or through intranasal sprays, transdermal patches, topical creams, or enemas. Small amounts of nicotine are found in foods such as tomatoes, potatoes and eggplant. Nicotine can be absorbed into the bloodstream through the mouth, lungs, bladder, gastrointestinal tract, and skin. From 70 to 80% of nicotine absorbed in the gastrointestinal tract is converted into its most important metabolite, cotinine, during its first passage through the liver [1]. The rate of nicotine absorption through the skin and mucous membranes is directly dependent on its concentration. The half-life of nicotine is approximately two hours [2]. From a dermatological point of view, tobacco use carries many dangers to the skin, which can occur both through a direct effect on the epidermis and indirectly through the bloodstream. Tobacco smoke consists of a solid dispersed phase, which includes its main alkaloid - nicotine, as well as a volatile phase. It contains many mutagens and carcinogens, in particular polycyclic aromatic hydrocarbons (PAHs), nitrosamines and heterocyclic amines. The main toxic components of the solid phase include nicotine, phenol, catechol, quinoline, aniline, toluidine, nickel, N-nitrosodimethylamine, benzopyrene, benzanthracene and 2-naphthylamine. The main toxic components of the gas phase are carbon dioxide, carbon monoxide, hydrogen cyanide, nitric oxide, acetone, formaldehyde, acrolein, ammonium cations, pyridine, 3-vinylpyridine, N-nitrosodimethylamine and N-nitrosopyrrolidine. Gene expression studies in skin cells have shown that tobacco components activate the expression of 14 different genes involved in xenobiotic metabolism and response to oxidative or other stress [3]. The non-genomic effects of tobacco smoke are due to the formation of reactive oxygen species (ROS). Nicotine and its metabolites affect the skin through activation of nicotinic cholinergic receptors (nAChRs) expressed by epithelial cells [4]. Signs of smoking on mucous membranes and skin Heavy smokers, as a rule, can be identified by characteristic manifestations on the skin and mucous membranes. Yellow discoloration of light-colored mustaches and nails soaked in tobacco byproducts is often found. When smoking cessation occurs, a distinguishable boundary appears between the distal pigmented portion of the nail and the growing normal nail, a phenomenon known as the Harlequin nail or quitter nail (Figure 1). Based on the length of a normal nail that has grown, one can guess how long a person has not smoked. Chronic heat from a lit cigarette also stains the fingertips due to post-inflammatory hyperpigmentation. One third of smokers have noticeable perioral hyperpigmentation [5]. In children who passively inhale tobacco smoke [6], as well as consuming nicotine sublingually, gum pigmentation can be detected [7]. Pigmentation of the gums is caused by the formation of melanin granules synthesized in melanosomes. Nicotine, which can act as a precursor for melanin synthesis, can irreversibly bind to melanin and accumulate in melanin-containing tissues [8]. It is known that other components of tobacco, especially carcinogens such as N'-nitrosonornicotine (NNN), 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and benzopyrene, accumulate in tissues containing melanin [9].
Figure 1 | Harlequin's nail or "one who is trying to get off"
(a) Nicotine staining and line of demarcation on several fingers. (b) A line of demarcation between the distal pigmented part of the nail and the newly formed proximal pink part of the nail appeared after abrupt cessation of smoking.
Smoking can also affect the tongue, palate and other anatomical structures of the oral cavity. In smokers, you can find characteristic hyperplasia of the papillae and black pigmentation of the dorsal surface of the tongue, the so-called. "black hairy" tongue. Smoker's tongue (or nicotine leukoplakia of the tongue, leukokeratosis nicotina glossi) is a homogeneous keratosis with inflamed salivary glands that look like papules with a depression in the middle. Smokers of bidis (tobacco wrapped in Cromandel ebony leaf) experience central atrophy of the tongue papillae, which is reversible upon cessation of smoking. Smoker's palate (or nicotine leukoplakia palati, leukokeratosis nicotina palati) is an asymptomatic homogeneous keratosis of the posterior two-thirds of the hard palate associated with multiple red, centrally depressed papules representing inflamed salivary glands. This can only be found among smokers, especially among avid pipe lovers. On the other hand, tobacco chewers develop a white discoloration at the site of contact with the tobacco, which may ulcerate (Fig. 2)
Figure 2| Keratosis caused by chewing tobacco (smokeless tobacco keratosis)
Hyperkeratosis of varying severity developed at the site of contact of the mucous membrane with tobacco. Grade 1 (a) mucous membrane of normal color with slight superficial wrinkles. Wrinkles disappear when the mucous membrane is stretched. Grade 2 (b) a combination of white-gray and reddish areas with moderate wrinkles. When stretched, neither wrinkles nor color disappear. The different thickness of wrinkles of more severe grade 2 (c) is noteworthy. Grade 3 (d) is similar in color to the more severe grade 2 (white-gray and reddish), but the mucous membrane shows deep, thick wrinkles that do not disappear with tension. The longer the habit, the higher the degree. The patient in photo (d) has an area of ulceration at the top of the mucosal area in contact with tobacco. Quitting the habit, especially if it lasts for a short period of time, leads to regression of damage.
Premature aging of the skin One of the most characteristic and socially significant manifestations of smoking is premature aging. The “smoker’s face” is typically mottled with pronounced wrinkles, facial features become thinner, emphasizing the bone relief, the skin is atrophic with a grayish tint against the background of plethora [10]. The degree of change is directly related to the number of cigarettes smoked per day (Fig. 3). Premature aging is more pronounced in women than in men. The severity of wrinkles is enhanced by direct contact with cigarette smoke, which dries out the stratum corneum of the epidermis and causes a moderate inflammatory reaction [12]. In vivo experiments in mice also show that passive exposure to tobacco smoke leads to premature skin aging [13]. Skin that is exposed to tobacco smoke is thin and fragile and tends to sag. While tobacco smoking itself is an independent risk factor for wrinkle formation, exposure to sunlight enhances this effect [14]. Smoking also affects skin that is protected from the sun. The best predictors of aging are the number of cigarettes smoked per day and age.
Figure 3 | Manifestations of smoking on the face of a smoking twin
Noteworthy are the significant differences in the appearance of smoking twin 1 compared to non-smoking twin 2. Twin 1, according to anamnesis, has about 52.5 pack/years (the number of packs of cigarettes smoked per day multiplied by the total duration of smoking in years). The total lifetime insolation of twins is comparable.
Cigarette smoke is an important factor in accelerating the aging process due to the formation of free radicals and the development of corresponding pathological processes (Fig. 4). Smoking can wear down cellular defense and repair mechanisms, leading to the accumulation of damage associated with mutations and defective proteins. Smoking also disrupts the renewal of intercellular substance in the skin [17], which leads to an imbalance between the processes of synthesis and degradation of proteins in the connective tissue of the dermis. In addition to the fact that smoking reduces the synthesis of collagen types I and III (an important condition for accelerated skin aging) [18], it also increases the content of tropoelastin and accelerates collagen degradation [19]. Smoking is an independent risk factor for an increase in the number of elastic fibers of the papillary dermis in skin not exposed to direct contact. This increase is a consequence of increasing degradation of the elastic substance, similar to solar elastosis [20]. Disturbances in the intercellular substance of connective tissue may contribute to the molecular mechanisms of premature skin aging in smokers. In vitro experiments show that collagen biosynthesis in cultured fibroblasts is significantly reduced after exposure to tobacco smoke extract [18]. Smoking increases the expression of matrix metalloproteinases (MMP-1, MMP-2, MMP-3, MMP-7, and MMP-8) [17,19]. In the skin of smokers, compared to non-smokers, the expression of MMP-8 is increased, and the expression of TIMP-1, an MMP inhibitor, is decreased. Increased expression of MMP-1 in active smokers is also detected in areas of the skin protected from the sun. In vitro experiments have shown that tobacco smoke extract can enhance the expression of MMP-1 and MMP-3 in dermal fibroblasts. Moreover, MMP-1 expression is significantly increased in fibroblasts after stimulation with tobacco smoke extract and UVA1 ultraviolet irradiation, independently of each other [18].
Figure 4 | The mechanism of premature aging caused by smoking
Smoking increases the amount of ROS and MMPs, which leads to collagen degradation and disruption of the structural integrity of the extracellular matrix. This reduced mechanical pressure on fibroblasts leads to an increase in intracellular oxidant levels - this, in turn, stimulates MMP-1 expression even more. FB - fibroblasts; ROS - reactive oxygen species.
Transforming growth factor (TGF)-b1 likely plays a role in age-related skin changes associated with tobacco smoking. Tobacco blocks the cell response to TGF-b by negatively regulating the TGF-b1 receptor and inducing non-functional forms, suggesting a role for TGF-b1 modulation as a possible way to inhibit premature skin aging [22]. Nicotine may impair dermal remodeling by increasing the activity of cell cycle regulators, apoptosis, and the amount of collagen Ia1, elastin, and MMP-1 [23]. By changing the metabolism of intercellular substances and nAChR expression, it can influence physiological aging. Nicotine also promotes elastin overproduction and keratinocyte nAChR-α7 overexpression. However, the nicotinergic effect on dermal fibroblasts is mainly mediated by the nAChR-a3 subtype [24]. Skin cancer Epidemiological studies have identified both positive and negative relationships between smoking and the incidence and aggressiveness of certain types of skin cancer. The development of smoking-induced skin cancer is explained by the following mechanisms: accelerated aging, mutagenesis, stimulation of tumor cell growth and invasion, neovascularization and stromal remodeling. Despite the fact that there are studies that did not find any relationship with smoking, [25] most studies have found that smoking is an independent risk factor for the development of squamous cell carcinoma (SC), in particular, SC of the penis, vulva, cervix and anus [ 26]. Quitting smoking reduces these risks [27]. Smoking increases the incidence of highly aggressive forms of vulvar PR and reduces survival. Women who smoke and have genital warts are 35 times more likely to develop vulvar cancer, suggesting a synergy between smoking and genital warts [27]. A significant relationship between malignant neoplasms of the oral cavity and smoking has also been established [28]. Smoking unfiltered cigarettes further increases the risk [29]. Women who smoke are particularly susceptible to oral malignancies [30]. Most studies have not found a clear association between smoking and the risk of basal cell carcinoma (BCC) [26], but the data are conflicting. Cigarette smoking may be associated with a predominance of larger BCCs (>1 cm in diameter) [31] and more aggressive forms [32]. Earlier studies that examined the relationship between smoking and melanoma found that the number of men who smoked was in remission five years after diagnosis was significantly lower than that of non-smokers [33]. Smokers are more likely to have early and visceral metastasis [34]. More recent studies, however, show that smoking habit has no effect on the risk of malignant melanoma [26]. Long-term smoking may even reduce the risk of developing melanoma, especially acral melanoma [35]. The protective effect of smoking on cutaneous melanoma may be associated with the participation of nicotine in melanin synthesis, its affinity for melanin-containing tissues, irreversible binding to melanin, and the deposition of tobacco carcinogens NNK and NNN in tissues containing melanin. It is noteworthy that using the example of hairless mice, the inhibitory effect of tobacco components, caused by a block of the nuclear factor-jB signaling pathway, on skin tumors induced by UVB irradiation was demonstrated [13]. Smoking is associated with a reduced risk of classical Kaposi's sarcoma (cKS) [36] and AIDS-associated Kaposi's sarcoma. However, in clinical trials, transdermal nicotine in the treatment of KS in non-smokers was ineffective against KS lesions or HHV-8 viral load [38]. The carcinogenicity of tobacco is mainly associated with the action of PAHs, nitrosamines and heterocyclic amines. It is known that smoking slows down the repair of single-stranded DNA breaks [39] and also has a direct oncogenic effect [40]. The pro-tumorigenic effect of nicotine is also explained by an increase in the frequency of mitosis of cells in the basal layer and the appearance of hypertrophied epithelial cells in the epidermis [41]. Nicotine and nitrosamine contained in tobacco promote tumor growth through the positive regulation of cellular nAChRs, which induces the invasion of malignant cells and inhibits apoptosis [42]. The proinvasive effects of nicotine are due to nAChR-α7 [43]. Nicotine increases the secretion of acetylcholine, as well as the expression and activity of nAChRs in malignant cells [44]. Receptor-mediated toxicity of tobacco is the result of the interaction of the downstream signaling pathways Ras/Raf-1/MEK1/ERK, as well as JAK-2/STAT-3, starting with the activation of nAChR-α7 keratinocytes (Fig. 5) [45]. Nicotine has also been shown to stimulate tumor growth by increasing the activity of phospho-ERK (phosphorylated extracellular signal-regulated kinase). The proliferative effects of nicotine can be prevented by using a high-affinity nAChR-α7 blocker, α-cobratoxin [46]. Pharmacological block of nAChR-α7 disinhibits apoptosis and reduces the expression of vascular endothelial growth factor (VEGF), which leads to inhibition of angiogenesis [47].
Figure 5 | Interaction of the downstream signaling pathways Ras/Raf/MEK/ERK and JAK-2/STAT-3 with nAChR-α7 of keratinocytes
Stimulation of nAChR-α7 by both the physiological ligand acetylcholine and nicotine leads to changes in gene expression through transactivation of STAT-3. Transactivation can occur through two signaling pathways associated with nAChR-α7. The pathway of sequential activation of Ras/Raf/MEK/ERK leads to an increase in the concentration of STAT-3 in the cytoplasm, due to the positive regulation of expression, while activation of the tyrosine kinase JAK-2 causes phosphorylation of STAT-3 with subsequent translocation of STAT-3 dimers into the nucleus, where there will be a change in gene expression.
Indirect oncogenic effects of nicotine associated with endothelial cells and fibroblasts of the dermis are also known. Nicotine can enhance tumor growth by enhancing tumor vascularization and creating a pro-carcinogenic environment in the stroma [49]. The effect of smokeless tobacco extract on dermal fibroblasts enhances the production of factors that accelerate the proliferation and germination of immortalized keratinocytes. Thus, ultraviolet light and nitrosamines from tobacco can induce carcinogenesis, while nicotine promotes the growth and proliferation of altered cells. Impaired wound healing Smokers have an increased risk of complications from surgical wounds [50]. Animal studies have shown that smoking within eight weeks before surgery increases the likelihood of skin flap necrosis [51]. At the same time, abstaining from smoking reduces the likelihood of infectious complications [52]. The pathobiological basis for changes in wound healing caused by tobacco are similar to those associated with premature skin aging. For example, collagen production is also reduced and intercellular metabolism is altered, with MMP-8 levels increasing. It has been shown that this contributes to disruption of regeneration processes. It is likely that smoking also affects wound healing by inhibiting the migration of fibroblasts to the wound bed, which leads to their accumulation at the wound edge. Increasing the lifespan of fibroblasts simultaneously with decreasing their migration may promote fibrosis and excessive scarring [54]. It is important that epithelization of the wound is caused by the migration of keratinocytes over the exposed dermis. Keratinocyte migration is regulated by both nicotinic and muscarinic subtypes of ChR. It can be inhibited by activation of nAChR-α7, which are responsible for coupling ion flows with cascades of protein kinase signaling pathways that regulate integrin expression and Rho kinase activity [55–58]. Smoking may slow wound healing indirectly through slowing blood flow. While smoking reduces blood flow, oxygen concentration, and aerobic metabolic activity in the skin, nicotine itself increases tissue oxygen content, even despite reduced blood flow [59]. Most likely, the effect of smoking on microcirculation is not due to the effects of nicotine. It is likely that nicotine affects regeneration only in toxic doses. In the therapeutic range, however, transdermal nicotine has been shown to be different: it normalizes microvascular perfusion of the digital skin [60] and stimulates wound healing and angiogenesis [61]. In an experiment on mice, nicotine significantly promoted regeneration [62]. In addition to the positive regulation of the rate of epithelialization, the mechanism is probably associated with increased synthesis of type I collagen [63]. Chronic dermatoses affected by smoking Contact dermatitis Cigarette smoking and snuff use are proven risk factors for the development of allergic contact dermatitis. The following allergens are found in cigarettes: cocoa, menthol, licorice, rosin and formaldehyde [64]. Smoking dose-dependently increases the likelihood of a positive skin test for nickel [65]. Nicotine, itself an allergen, in skin tests can cause allergic or simple contact dermatitis at the site of application [66]. Atopic dermatitis Although smokers have significantly higher serum IgE titers than nonsmokers, evidence regarding the effect of smoking on atopic dermatitis is inconsistent. Some studies report that smoking is an independent risk factor for hand eczema [68]. However, other studies have not found such a relationship between smoking and atopic dermatitis or hand eczema [69]. Psoriasis A significant direct correlation between psoriasis and smoking, depending on the number of cigarettes smoked per day, is well known [69]. Smoking is an independent risk factor for psoriasis [70], more dangerous in men [71]. Moreover, periods of remission after treatment are shorter in smokers [72]. Palmoplantar pustulosis (PPP) PPPP (Figure 6) is one of the most common inflammatory skin diseases associated with smoking. About 95% of all patients with DILI are smokers, the majority are heavy smokers [73]. Quitting smoking significantly improves the condition: fewer pustules, less area of erythema with scaling [74]. The target of inflammation in DILI is the acrosyringium (Intraepidermal part of the sweat gland duct - editor's note). Due to impaired expression of nAChRs and other components of the skin cholinergic system, patients with DILI may develop an abnormal inflammatory response to nicotine. These observations indicate a role for nicotine in the pathogenesis of DILI [75].
Figure 6 | DILI, also known as “pustular psoriasis”
Cutaneous lupus erythematosus Studies have found a statistically significant association between active smoking and the development of cutaneous lupus erythematosus [76]. An association between discoid lupus erythematosus (DLE) and smoking has also been shown. Smokers with DLE have more extensive lesions at disease onset [77]. Also, the effectiveness of antimalarial drug therapy is reduced by smoking [78]. Hair and hair follicle diseases Smoking is associated with premature graying of hair in both men and women, as well as earlier hair loss in men [79]. The course of androgenetic alopecia is aggravated by frequent smoking, and the onset occurs earlier, the higher the dose [80]. In vivo studies have shown that in mice exposed to tobacco, epidermal atrophy develops, subcutaneous fat tissue becomes thinner, and the number of hair follicles decreases. Cellular apoptosis is also observed in the hair follicle [81]. Smoking affects the microvasculature of the hair papilla in the dermis and leads to DNA damage in hair follicle cells [82]. It also unbalances the protease-antiprotease system, leading to changes in the hair growth cycle. An increase in the concentration of proinflammatory cytokines causes microinflammation and fibrosis of the follicles. Pustular dermatoses While some studies suggest a dose-dependent linear relationship between the incidence and severity of acne vulgaris and tobacco smoking [83], other studies have not revealed such a relationship [84]. In contrast, a lower prevalence of acne has been reported among smokers [85]. The risk of developing rosacea is also lower among smokers, probably due to the vasoconstrictive effects of smoking [86]. A clear relationship has been established between smoking and hidradenitis suppurativa (the so-called acne inversus) [87]. The key element in the pathogenesis of hidradenitis suppurativa is probably choline-sensitive cells of non-nervous origin, as evidenced by the abnormal expression of nAChRs in the skin involved [88]. Pemphigus vulgaris It is noted in the literature that pemphigus vulgaris develops more favorably when smoking. This is consistent with data that among patients with pemphigus, smokers make up a minority [89]. Smokers with pemphigus vulgaris go into partial and complete remission more often than non-smokers [90]. There is also an inverse relationship between tobacco smoking and dermatitis herpetiformis and associated celiac disease [91]. Smokers with newly diagnosed celiac disease are less likely to have endomysial antibodies than nonsmokers [92]. However, one study did not find any association between smoking and the presence of antigliadin antibodies [93]. No significant relationship was found between smoking and mucosal pemphigoid [94]. Skin ulcers Smoking is a risk factor for the development of pressure ulcers [95] and infected ulcers in patients with diabetes [96]. More than 90% of patients with ulcerative lesions due to Buerger's disease (thromboangiitis obliterans) are smokers. Long-term remissions are possible when smoking is stopped, while relapses are caused by a return to the bad habit [97]. Viral skin diseases Smoking is known to increase the risk of genital warts in men [98]. But the incidence of recurrent herpes labialis in smokers, on the contrary, is much lower, especially when smoking a pipe [99]. This is most likely due to the ability of tobacco to inhibit the replication of the herpes simplex virus and significantly reduce its cytolytic effect [100]. However, more recent studies have shown that nicotine applications via a transdermal patch induce herpes simplex virus reactivation as well as viral shedding in rabbits carrying the infection [101]. Other diseases of the skin and mucous membranes A significant association has been established between smoking and diabetic necrobiosis lipoidica [102]. Smoking has also been associated with generalized urticaria [103]. Men who smoke cigarettes or beedis are at significantly higher risk of developing arsenic-related skin lesions than non-smokers [104]. On the other hand, there is an inverse relationship between tobacco smoking and geographic language [105]. Skin diseases treated with nicotine As noted above, smoking can aggravate the course of certain diseases, promote carcinogenesis and potentially lead to addiction. However, no matter how paradoxical it may be, in the case of many dermatological diseases, nicotine shows itself to be an effective monotherapy. Buerger's disease is clearly associated with smoking but is successfully treated with nicotine-containing chewing gum (Fig. 7) [106]. It is likely that other components of tobacco induce vasospasm and aggravate thromboangiitis obliterans, but the use of nicotine may have valuable therapeutic potential. In addition, a key component of treatment for Buerger's disease is smoking cessation.
Figure 7 |Buerger's disease during nicotine treatment
(a) Left big toe with a large deep ulcer (1.5–2.0 cm) in a 38-year-old man who has been smoking for 20 years. (b) The ulcer healed 20 days after starting treatment with nicotine chewing gum (2 mg per piece) given twice daily. At least 14 months later, while taking 2 nicotine gums per day without other medications, the patient's legs remained in good condition, with no ulcers or intermittent claudication.
The therapeutic effect of cigarette smoking on aphthous ulcers of the oral and genital mucosa in Behçet's disease is also known [107]. Accordingly, cessation of smoking leads to the appearance of mucocutaneous manifestations, especially aphthous stomatitis. However, when treated with nicotine, aphthous ulcers resolve within a few days [108]. The mechanism of this action is likely related to the ability of nicotine to inhibit the release of proinflammatory cytokines IL-6 and IL-8 from keratinocytes and endothelial cells in the dermis. Tobacco smoke extract also reduces IL-8 release but increases VEGF production in human keratinocytes [109]. Inflammatory bowel diseases (IBD), according to epidemiologists, are associated with smoking [110]. Smoking and/or consumption of pure nicotine can affect such mucosal and skin manifestations of IBD as aphthous stomatitis, pyoderma gangrenosum and erythema nodosum. Most patients with ulcerative colitis are nonsmokers or have stopped smoking [111]. Smoking cessation worsens the progression of ulcerative colitis (UC), but progression slows again when smoking resumes. In contrast, patients with Crohn's disease who smoke tend to have a more severe course [112]. Nicotine is a key component of tobacco that has an effect on IBD, as its administration through transdermal patches is known to inhibit inflammation in UC, which is not observed in Crohn's disease [113]. Interestingly, mice with trinitrobenzene sulfonic acid-induced colitis that received the nAChR-α7 agonist anabasine had less tissue damage than mice that did not receive it [114]. Nicotine has also been used in the treatment of pyoderma gangrenosum and erythema nodosum, probably associated with IBD, and in the treatment of malignant atrophic papulosis (so-called Degos disease) [117], Kimura disease [116] and eosinophilic pustular folliculitis [118]. Similar to ulcerative colitis, recurrent aphthous stomatitis (RAS) is less common in smokers [119]. It is known that aphthous stomatitis also suddenly worsens with a sudden cessation of smoking, and weakens when resumed. Nicotine replacement therapy significantly reduces the relapse rate of ASD, suggesting that nicotine could be used as a drug [120]. Although published studies do not suggest an association between smoking and lichen planus (LP) of the oral mucosa, there is one case of otherwise resistant LP that responded to nicotine gum [121]. Transdermal nicotine has been shown to directly reduce inflammation in the skin [122]. Nicotine and other nAChR agonists may exert anti-inflammatory effects by inhibiting lymphocyte proliferation, endocytosis and phagocytosis by dendritic cells, as well as the production of ROS, superoxide and pro-inflammatory cytokines [123]. It is likely that the mechanism of anti-inflammatory action is also due to anti-inflammatory cytokines, IL-10 and TGF-b, glucocorticoids and soluble receptors that neutralize the effects of pro-inflammatory cytokines. Nicotine stimulates nAChR-α7, which modulate inflammation in the absence of parasympathetic innervation. This is an essential receptor necessary for the inhibition of cytokine synthesis during the implementation of cholinergic anti-inflammatory pathways [124]. On the contrary, the absence of nAChR-α7 leads to an increase in the pro-inflammatory cytokine response [125]. Conclusion Tobacco and its components affect the skin directly and indirectly. Various skin cells express nAChR subtypes, the activity of which influences cell viability and function. Changes in the skin are caused by both genomic and non-genomic effects of tobacco toxins and nAChR agonists. The effects are varied: from accelerating the aging process to mutagenesis. Epidemiological studies have established both positive and negative relationships between tobacco smoking and various skin diseases. A positive relationship with certain diseases may have a beneficial effect on their course upon smoking cessation, while the opposite may require specific treatment with nicotine agonists. It is important to understand the difference between the effects of tobacco and pure nicotine in Buerger's disease. Part of the anti-inflammatory effect of nicotine is due to stimulation of nAChR-α7. The effects of tobacco components and pure nicotine are summarized in Table 1. Further research is needed on nicotinergic substances as potential therapeutic agents for certain skin diseases. Table 1 | Generalized effects of tobacco/nicotine on skin
Treatment
To get rid of unsightly deposits, unpleasant odors and other symptoms, you need to determine their cause. To do this, you need to contact a therapist who will conduct an examination and prescribe additional tests and examinations (general analysis and blood biochemistry, throat culture, gastroscopy, ultrasound, coprogram, etc.). Based on the results, he will recommend therapy, diet and, if necessary, give a referral to a gastroenterologist, infectious disease specialist, dentist or other specialist.
After treating the root cause of plaque, its intensity should decrease. But to speed up the process, doctors recommend paying more attention to oral hygiene, brushing the surface of the tongue twice a day with a soft-bristled toothbrush (but not a scraper, so as not to cause injury), drinking more water and rinsing your mouth with herbal decoctions.
Prevention
It is not always possible to prevent the formation of plaque on the tongue, especially if it is a symptom of an infectious, viral or fungal infection. But you can reduce the likelihood. The recommendations will not be new: you need to adhere to a healthy lifestyle, do not smoke, do not abuse alcohol, drink coffee and tea in moderation, exclude too fatty, spicy and fried foods, include more vegetables, fruits, whole grains in your diet, give preference to lean varieties of meat and fish and do not take medications (especially antibiotics) without a doctor's prescription.
What should be done to prevent plaque?
Prevention includes daily oral hygiene .
For preventive purposes, you can use mouthwashes with antibacterial properties .
Rinsing with a decoction of chamomile, sage and oak , as well as chewing propolis, are useful. These agents have a strong antibacterial and anti-inflammatory effect.
Rinsing with soda solution also gives a good effect.