DEKEMA
| The first Dekema kiln was developed under the direction of ceramic manufacturers back in 1930, and subsequently, in 1973, DEKEMA Service GmbH was founded in Freilassing, Germany. The robust design and reliable operation of the furnace were unsurpassed, which has become a tradition for the company. “It all started with a simple ceramic kiln. Today you receive a masterpiece." (Stephen Miller) “Reliability and consistency of results allow me to fully concentrate on my daily tasks.” (Marco Furle) AUSTROMAT oven range from |
Zirconium dioxide bridges – what are they and what are their features?
Article navigation
- What kind of material
- Zirconium dioxide bridge
- Advantages
- Are there any disadvantages?
- Indications and contraindications
- Manufacturing stages
- Popular manufacturers
- Service life and guarantees
- Habituation and care
- Price
Question for a specialist
With the invention of new materials and technologies for their processing, dental prosthetics has made a big step forward. Today, orthopedists are increasingly offering patients zirconium bridges - as durable as metal and aesthetically pleasing as ceramics. In today’s article we will tell you all about the features of a zirconium dioxide dental bridge, for what indications it is used and for whom it is contraindicated, and also dwell on other popular questions on the topic.
High-tech sintering furnace from
The catalog presents the following types of sintering furnaces for use in dental practice:
- Austromat 674 baSiC 2 is a sintering furnace for sintering milled ZrO2 workpieces. Up to 30 pieces can be made at a time, the design is equipped with three heating elements, the maximum temperature is 1530° C. Suitable for working with zirconium workpieces, as it does not discolor them.
- Austromat µSiC is a reliable dental furnace for sintering up to 120 units of zirconium at a time. Fast, energy-saving equipment with implemented environmentally friendly technologies. You can control the operation of the furnace remotely via the network and mobile devices.
- Austromat 664 is a high-temperature sintering furnace for sintering ceramics. The furnace is highly stable; sintering is carried out on 2 levels using micro-pearls. The design is equipped with two heating elements.
Sintering zirconium oxide and dioxide and producing crowns from this material is a complex process that uses a sintering furnace.
During the sintering process, a ceramic or metal frame is heated at a certain temperature. Gradually, the material is sintered and compressed. As a result, the semi-finished product acquires its final properties: strength and hardness.
Modern models of sintering furnaces allow sintering zirconium in an accelerated mode.
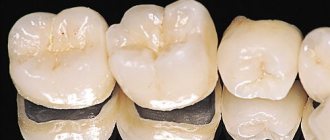
Zirconium dioxide bridge – what is it, types
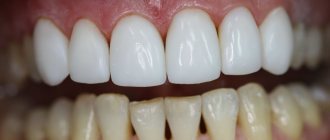
A zirconium dioxide dental bridge is a non-removable orthopedic structure that includes several crowns (0.5 mm thick) that is placed over abutment teeth or implants and restores missing teeth. Moreover, crowns are not created separately - they initially represent a single whole, i.e. bridge prosthesis. As a rule, in such a design there are no additional elements - acrylic gum and overlapping palate. But in exceptional cases, artificial gum can be built up along the edges of the crowns - when, for example, the patient has an uneven or unaesthetic natural gum level.
If we consider the types of zirconia bridges, we get the following classification:
- monolithic: made only from zirconium dioxide, without ceramic lining or painting,
- additionally colored zirconia bridges,
- classic or two-layer: zirconium dioxide frames, lined with ceramics to enhance aesthetics.
Advantages of zirconium dioxide sintering furnaces
- The models use new technology. It ensures minimal time for high-temperature firing and sintering. The preparation is completed in just 120 -180 minutes.
- Zirconium sintering furnaces are easy to operate. They are equipped with a special touch screen on which operating modes are switched.
- Modern technologies allow you to quickly connect the oven to a computer and quickly transfer all the necessary information.
Sintering furnaces have compact dimensions - this allows them to be located even in cramped laboratory conditions.
For more information, please contact the contacts listed on the website. Specialists will not only help you choose a furnace for dentistry, but will also provide prompt delivery of equipment in Moscow and other regions of the Russian Federation.
Advantages of metal-free ceramic bridges
Orthopedic dentists often recommend bridges made of zirconium dioxide to their patients, since this material gives the structures such positive qualities as:
- very high aesthetics and its long-term preservation (only with high-quality manufacturing): the material does not absorb dyes, less plaque settles on it than on tooth enamel,
- does not provoke “blueness” of the gums, like metal ceramics,
- biocompatibility, very low risk of allergies,
- the highest strength: several times higher than the strength of ceramics, and also stronger than some metal alloys,
- have a thinner layer of ceramics on top (compared to metal ceramics): because the metal is gray - under very thin ceramics it can be seen through,
- possibility of installation in any part of the row on natural teeth, as well as on dental implants,
- low thermal conductivity (compared to metal-ceramics): therefore, the supports under the prosthesis will not react so sharply to hot and cold food. But still, you shouldn’t get carried away with extremely hot and cold drinks / dishes, because... you can burn your gums and natural teeth,
- the supporting teeth do not need to be sharpened much,
- low risk of secondary caries and odor from under the crowns: from the inside, the structure fits very tightly to the supports, without gaps,
- very long service life: it is 20-25 years.
Zircon and zirconium
The zircon mineral is a zirconium silicate with impurities of copper, iron, calcium, zinc, uranium, thorium, hafnium and titanium. It occurs in nature in the form of prismatic crystals, aggregates, grains, and differs in color and transparency. Pure zirconium oxide is refractory, has low thermal conductivity, is slightly soluble in water, and is resistant to chemical reagents.
Zirconium is an element of group IV of the periodic table with an atomic mass of 91.224. It exists in two forms - crystalline and amorphous. The highest concentration of the substance is in alkaline rocks. In nature, it is found in combination with silicate oxide - the mineral zircon or as free zirconium oxide - the mineral baddeleyite.
What is zirconium oxide?
There are currently quite a lot of materials for making crowns.
One of them is zirconium oxide. This is a very popular modern ceramic material, which is the result of the introduction of high technology in the field of dentistry. It is the high strength, biocompatibility and aesthetics of zirconium oxide that have made it one of the most popular materials for dental prosthetics. The discovery of zirconium oxide is associated with the mineral zircon (Fig. 1), about which there are many ancient legends. More than three thousand years ago, on the island of Ceylon, zircon was used as an imperfect diamond and was used to make jewelry. The shiny stones were called “Matara diamonds”, since their source was one of the regions of Ceylon - Matara. There are several versions about who gave the modern name to the “imperfect diamond.” According to some sources, the German scientist Brückner in 1778 named it with the Arabic word “zarkun”, which means “mineral”. According to others, the discoverer of zircon is considered to be the chemist Werner (1783), who gave the mineral the name “tsargun” from two Persian words “tsar” - “gold” and “gun” - “color”. Still other sources claim that “zircon” is a modification of the common folk “jargon” - “deceiver”, i.e. "fake diamond" The mineral zircon began to be officially mentioned in scientific works in the 80s of the 18th century. In 1789, the German chemist Martin Heinrich Klaproth published the results of an analysis of a gemstone brought from the shores of Ceylon. During this analysis, a substance was isolated that Klaproth called “zirconium earth”; this was zirconium oxide.
Fig.1. Mineral zircon
There is currently some terminological confusion that needs to be cleared up. The zircon mineral Zirkon (ZrSiO4) is a zirconium silicate. Contains impurities of various metals. Based on color and transparency, the following types of zircon are distinguished: hyacinth - transparent, red, red-orange, red-brown, purple (Fig. 2); jargon - transparent, honey-yellow, smoky, colorless and starlite - transparent, blue. Zirconium oxide also occurs naturally as the mineral baddeleyite (Fig. 3).
Fig.3. Mineral baddeleyite
Zirconium Zirconium (Zr) is a chemical element of group IV of D. I. Mendeleev’s periodic table. Pure zirconium exists in two forms: the crystalline form is a soft, malleable metal of a grayish-white color (Fig. 4); amorphous form - bluish-black powder. It is not found in nature in its pure form; pure zirconium, free from impurities, was obtained only at the beginning of the 20th century.
Fig.4. Metal zirconium Zr
The minerals zircon and baddeleyite cannot be used in medicine due to the large number of impurities of various metals, which give them an unsuitable color for use, and impurities of radioactive metals (uranium, thorium). High-purity zirconium oxide is produced chemically. Zirconium oxide (dioxide) (ZrO2), in English-speaking countries the term Zirconia is acceptable, is one of the most refractory metal oxides, the melting point is 2715 °C. It exhibits amphoteric properties, is insoluble in water and aqueous solutions of most acids and alkalis, but dissolves in hydrofluoric and concentrated sulfuric acids, molten alkalis and glasses.
Zirconium oxide exists in the form of three crystalline phases: monoclinic (M), tetragonal (T) and cubic (C). During heating, zirconium oxide undergoes a phase transformation process, that is, a transition from one phase to another, and a change in volume occurs. To suppress phase transformations of zirconium oxide and obtain materials with the desired properties, stabilizing metal oxides are added to it, for example, oxides of calcium, magnesium, yttrium, and cerium. Depending on the amount of stabilizing agent, fully or partially stabilized zirconium oxide is distinguished.
The stable monoclinic phase (M) is found in nature in the form of the mineral baddeleyite, the metastable medium-temperature tetragonal (T) phase is part of zirconium ceramics, the unstable high-temperature cubic (C) phase stabilized by impurities of various metal oxides, due to its high refractive index and dispersion, is used in jewelry as an imitation of diamonds; in the USSR, such crystals were called cubic zirconia (Fig. 5), from the Physical Institute of the Academy of Sciences (FIAN), where they were first synthesized. But the name cubic zirconia is practically not used outside the former USSR and Eastern European countries. Abroad, this material is more often called jewalite and zirconite. In some cases, especially in translations from foreign languages, cubic zirconia is called zirconium or zircon (Fig. 6), which creates confusion, since cubic zirconia is a synthetic material imitating diamond, zircon is an unrelated mineral, and zirconium is a chemical element.
Fig.5. cubic zirconia
Fig.6. Types of cubic zirconia (cubic phase zirconium oxide)
The refractive index of cubic zirconia (2.15-2.25) is close to diamond (2.417-2.419), so it is difficult to distinguish them by eye. Due to this, they are often used in jewelry (Fig. 7). Gold jewelry can be decorated with cubic zirconia inserts. In costume jewelry, they are used to contrast the transparency or color of the main insert (the center of the composition), or as an imitation of diamonds. They are also used in dentistry for ceramic spraying and in the chemical industry. In addition, in dentistry they are used to decorate teeth - to make skyes (Fig. 8).
Fig.7. Jewelry with cubic zirconia
Fig.8. Skyes
In industry, zirconium oxide is widely used due to its unique properties (high hardness, low thermal conductivity, etc.). In the automotive industry, it is used to make brake discs for high-end sports cars. In the space industry, shuttle heat shields help ships withstand incredible temperatures. It is used in the production of refractories, technical ceramics, heating elements; enamels, glass, and cutting tools are made from it. Everyone is well aware of ceramic knives (Fig. 9), which have become firmly established in our everyday life. When heated, zirconia conducts current, which is sometimes used to produce heating elements that are stable in air at very high temperatures. Heated zirconium oxide is capable of conducting oxygen ions as a solid electrolyte. This property is used in industrial oxygen analyzers. It is also used in medicine, for example, as spherical heads of artificial hip joints (Fig. 10, 11). And finally, in dentistry, he can show all his best qualities in the role of dentures.
Fig.9. Zirconium Oxide Ceramic Knives
Fig. 10. Hip endoprostheses made of various materials
Fig. 11. Hip joint friction couple ceramic-zirconium oxide ceramic
Popular material manufacturers
Zirconium dioxide has been used for the manufacture of prosthetics for more than 20 years, but there are not many companies producing truly high-quality material. Let's consider the most famous brands that guarantee the highest quality:
- Prettau Anterior from Zirkonzahn (Italy): has a high degree of transparency, comes complete with liquids for coloring in natural shades in the laboratory,
- Katana Zirconia from Kuraray Noritake (Japan): comes in multi-layer discs with good clarity, easily dyed to any desired shade,
- IPS e.max ZirCAD MT Multi from Ivoclar Vivadent: polychromatic material has an almost natural gradation of transparency, but is only suitable for bridges with a length of 3 crowns,
- IPS e.max ZirCAD LT from Ivoclar Vivadent: a universal material that is used for bridges of 3 crowns or more and is also suitable for implant prostheses. Suitable for monolithic bridges and frameworks for ceramic veneering,
- Cercon xt / Cercon ht from Dentsply Sirona: This is a translucent zirconia stabilized with yttria, making it more durable,
- VITA YZ HT from Vita Zahnfabrik: characterized by good ability to transmit sunlight (almost like natural enamel). Easily painted in desired shades.
“I covered my front teeth with Prettau zirconium bridges. They look very good, for me it’s a perfect match in color)) And they even made the teeth a little longer. How they became when they were young, beautiful. I like everything, especially that the gums do not turn blue, and that they promise a very long service life. I hope that these bridges will last for a long time.”
Tatyana S., review from the website stomatology.rf
Zirconium fixation: basic and new concepts
Deca-methacryloyloxydecyl dihydrogen phosphate is something of a buzzword for those who are not chemists by trade, so in everyday life this adhesive monomer is simply referred to as MDP (an acronym formed using the initial three letters). This chemical agent was developed by Kuraray Noritake Dental in 1981 to improve the adhesion strength of hydroxyapatite, and has been indispensable in dentistry ever since. Fixation of zirconium restorations, by the way, is also impossible without MDP monomer.
Requirements for indirect restorations
Indirect restorations in modern dentistry must meet at least three requirements. First of all, they must ensure the preservation of tooth tissue. For any crown, as a rule, it is necessary to sacrifice approximately 70% of the enamel and dentin, and the feasibility of such a sacrifice can only be justified if a reliable prognosis for the functioning of the restoration is ensured. However, given that adhesive protocols are moving forward at tremendous speed, the amount of hard tissue reduction required today can be significantly reduced without compromising the reliability of the connection. In such cases, zirconium restorations can be used. The latter involve both sandblasting and the application of MDP adhesive monomer.
Durability is the second requirement for indirect restorations. This property of the restoration is largely related to the flexural strength parameters of the material used. And although zirconium is a fairly reliable material, the prognosis for its functioning is also significantly influenced by the method of fixing the restoration to the tooth. Last but not least important criterion is the aesthetic parameter of rehabilitation. From this point of view, metal-ceramic crowns are already a thing of the past, and all-ceramic structures are the gold standard today. The reputation of zirconium from an aesthetic point of view was somewhat “tarnished” by the excessive whiteness of the representatives of the first generations of this material. Now zirconium dioxide is available in different shades and there are even multi-layer types (KATANA Zirconia ML, STML and UTML, all Kuraray Noritake) that provide the desired aesthetic result. Zirconium sintering remains a possible veneering option and is the most commonly used. A multilayer restoration helps to achieve different shades of transparency in the area of the incisal edge of the structure and its opacity in the cervical area: thus, light passes through the incisal part of the restoration, but is blocked at the neck of the tooth. Materials such as KATANA Zirconia ML also make it possible to maximally imitate natural tissues in the area of the body of the restoration, optimizing the process of their selection: for example, when restoring the neck with body shade A1, the transparency of this increases to the area of the incisal edge by exactly two transition shades.
Photo 1. KATANA Zirconia UTML
Photo 2. KATANA Zirconia STML
Photo 3. KATANA Zirconia ML
Photo 4: The veneers were made from KATANA Zirconia UTML and stained using CERABIEN ZR external stain (Kuraray Noritake).
Surface
Using the example of Daniele Rondoni from Savona (Italy), one can trace how the world of dental technicians is changing when using zirconium materials instead of their ceramic counterparts. According to the Rondoni philosophy, the initial selection of restorative materials should be wide enough to allow specific individual solutions to be realized, while the use of ceramics can be combined with lithium disilicate or zirconia copings, which helps to maximize the effect of a natural imitation of the restoration. Using sintered ceramic, the technician can modify the texture of the restoration to give it age-appropriate parameters. Regarding the surface structure, thorough polishing of the occlusal surface is the best prevention of abrasion of antagonist teeth and helps maintain optimal occlusal balance. Thus, the hardness of the material fades into the background, and the first is occupied by the parameters of smoothness and resistance of the material.
Flexural strength
When choosing a material for restorations, the dental technician will choose the multi-layer version of KATANA Zirconia Ultra Translucent Multi-Layered (UTML) for veneers or crowns in the anterior area. The transparency of this zirconium sample is comparable to that of glass.
This aspect is extremely important for the restoration of incisors and canines of both jaws. A crown made of KATANA Zirconia UTML harmonizes perfectly with adjacent natural teeth also due to the fact that this type of material is not excessively white. The second generation zirconium dioxide aesthetic material is sintered at a temperature of 1550° C, this temperature is maintained for 2 hours. The dental technician must be aware of the difference in the above mentioned temperature and setting for KATANA Zirconia High Translucent Multi-Layered at 1500 °C.
For larger bridgework, it is the last mentioned zirconium material that should be used, as KATANA Zirconia Super Translucent Multi-Layer (STML) is designed for the production of prosthetic structures up to a length of 4 tooth units. KATANA Zirconia UTML can be used for small anterior fixed prostheses, but is more suitable for single bridges and veneers.
The reason for this narrow application is that the flexural strength of these highly aesthetic zirconia materials is lower than that of standard zirconia - 1125 MPa, which is suitable for the manufacture of fairly large prosthetic elements. The flexural strength of highly esthetic zirconia varieties (approximately 750 MPa [STML] and 550 MPa [UTML]) is sufficient to ensure long-term performance of single esthetic restorations and limited span bridges.
Preparation
Flexural strength is not the only decisive factor in ensuring the functional reliability of restorations; other properties of the material are also of great importance, as are the specific stages of preparation for fixation of restorations. The preparation of teeth should be carried out with the formation of a champ, without any sharp edges and deep shoulder-type ledges. Obviously, it is also advisable to avoid any kind of undercutting. Since the restorations are fixed adhesively, it is recommended to avoid the presence of preparation channels, and all edges should be carefully rounded. When preparing for a full crown, it is necessary to ensure that the height of tissue reduction on the palatal and vestibular sides is strictly the same. When preparing for veneers, the amount of enamel reduction in the area of the incisal edge and neck should not exceed 0.4-0.8 mm, and on the labile side - no more than 0.5 mm. For inlays, it is enough to remove about 1 mm of tissue thickness, and when making full crowns, a similar space parameter should be provided on the lateral sides of the restoration.
Minimum wall thickness for restorations made of KATANA material
Maintain a pressed porcelain thickness of 0.8 mm in all areas of the tooth. When finishing a zirconium frame, its thickness must be at least 0.4 mm.
Fixation
Many options and modifications have been proposed for fixing zirconium structures. According to Professor Matthias Kern from the University of Kiel in Germany, further research in this area is generally no longer justified. As a scientist and practitioner, Kern has been involved in the development of the zirconia cementation protocol for the past 20 years. Based on his extensive experience, the scientist is convinced that in order to achieve reliable cementation of zirconium dioxide, three basic requirements must be met: firstly, fixation without a rubber dam is not fixation; secondly, the need to achieve micromechanical retention by sandblasting; thirdly, the need to ensure chemical communication. Based on extensive research, Kern is convinced that chemical adhesion can only be achieved using MDP monomer. His first publication on this topic dates back to 1998, and described his experience using PANAVIA (Kuraray Noritake), containing MDP monomer, to ensure adhesion of zirconia after sandblasting restorations.
Sandblasting
Dentists and dental technicians are trying to find an alternative to sandblasting zirconium, but attempts at such research remain only attempts. It was proposed to attach a layer of silica to the zirconia, which would strengthen the bond of the restoration, but, according to Kern, these and similar innovations such as the Rocatec method were unsuccessful. Silanization of zirconium is also ineffective because the material does not react with silane. Therefore, sandblasting is indispensable. The latter can be carried out in a small chamber, providing a slight air pressure of 0.5 bar for a soft type of abrasion, and 2.5 bar for a hard one. However, the pressure parameter is not key. Kern recommends sandblasting at 1 bar pressure to ensure proper surface roughness. It is obvious that the outer part of the restoration should be protected as much as possible from the influence of abrasive particles. It is also worth applying a dye (waterproof marker) to the surface being treated, which begins to disappear during sandblasting, providing more complete control over the abrasion process.
Photo 5: Low pressure sandblasting of zirconium oxide is essential for effective adhesion.
Adhesive monomer
After sandblasting the surface of the restoration, it can be cleaned using alcohol, although this step is optional. If alcohol is contaminated with saliva residues, its effect can be considered zero. However, the key is to ensure that the zirconium is fixed using a material containing MDP monomer. The latter is absent in glass ionomer cements, which are also sometimes used for cementing esthetic restorations due to their ease of use. Kern does not recommend the use of such materials. Research results indicate that composite cements containing MDP monomer provide the most long-term results in the functioning of zirconium structures. The oldest known cement in this category is PANAVIA EX, which was introduced to the market back in 1983. The optimized version of PANAVIA V5 was recently introduced into practice as the only cement that can be used absolutely for all types of cementation. All cements and bonding agents manufactured by Kuraray Noritake contain MDP monomer.
It is somewhat ironic that Kern, conducting its research in the state of Maryland in the USA for two years, recorded remarkable results in the functioning of Maryland-type adhesive bridges after appropriate processing and bonding. It has also been found that the retention of such structures is more durable when using a restoration design with only one retaining wing. For example, if an adhesive zirconia bridge with one retention wing is cemented using cement containing MDP monomer to replace, for example, an upper lateral incisor, the favorable prognosis for such a design can be up to 20 years. The success rate of such restorations over 5 years is 95.2%; similar results are typical for classic zirconium bridge structures.
Thus, sandblasting and the use of MDP monomer are mandatory steps to ensure strong adhesion of zirconia restorations. In addition, the MDP agent itself is an extremely long-lasting chemical that enhances the results of prosthetic rehabilitation for patients.
Source: stomatologclub.ru
Zirconium oxide in dentistry
Due to its unique properties, zirconium oxide is used in dentistry for the manufacture of: prosthetic frames, monolithic crowns, pins, stump inlays, fixed splints, fixed prostheses supported by implants (Fig. 12-15).
Fig. 12. Crowns and bridges
Fig. 13. Stump inlays
Fig. 14. Individual abutment on a titanium base and crown on an implant
Fig. 15. Prosthesis on implants
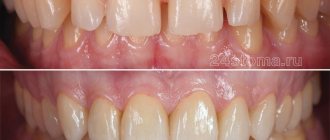
More recently, crowns and bridges on a metal frame were considered the generally accepted standard and no alternative method of treatment in dentistry, metal provided strength, ceramics - aesthetics. However, metal-ceramic restorations looked monochrome and opaque (Fig. 16), with a dark gray gum line (this effect is achieved by a metal frame). In dentistry, extensive research and searches have been carried out for materials suitable for dental prosthetics, which are aesthetically acceptable, have sufficient strength, wear resistance and at the same time are biocompatible - well tolerated by the human body. A high-tech solution was found with the introduction of zirconium oxide.
Fig. 16. Advantages of zirconium oxide crowns
This material is not inferior in strength to metal, has the properties of translucency and light transmission, which allows you to convey the natural color and transparency, like that of a natural tooth (Fig. 16). The metals from which prostheses are made sometimes cause allergic reactions and galvanism in the patient. Therefore, crowns based on zirconium oxide are one of the options for people with hypersensitivity and intolerance to metals and acrylates. The smooth surface prevents the accumulation of plaque, which promotes good oral hygiene and such crowns are not stained.
Due to the exceptional strength and optical properties of zirconium oxide, crown thickness can be less than that of all-ceramic or metal-ceramic restorations. This allows for a more gentle grinding of teeth and ensures the preservation of a larger volume of healthy tooth tissue. Therefore, when using prosthetics with zirconium crowns, there is a lower probability of nerve removal. The low thermal conductivity of the material ensures that there is no discomfort when eating hot or cold food in the presence of “live” teeth.